Abstract
Background
Prostate cancer (PCa) is the most common malignancy diagnosed among men after lung cancer in developed countries. Investigation of the underlying molecular mechanisms of PCa is urgently needed in order to develop better therapeutic strategies and to reveal more effective therapeutic targets. In this study, we aimed at exploring the potential functions of CASC11 in association with miR-145 and IGF1R during the malignant progression of PCa cells.
Methods
We initially investigated the oncogenic potential of noncoding members of CASC gene family and analyzed the effects of CASC11 overexpression on proliferation, migration, and colony formation ability of DU145, LNCaP, and PC3 PCa cells. We, then, exprlored the association of CASC11, miR-145, and IGF1R expression and their impacts on PI3K/AKT/mTOR signaling pathway in in vitro models.
Results
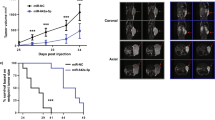
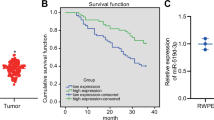
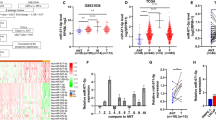
In silico analysis revealed that of the CASC family only CASC11 showed consistent results considering its differential expression as well as its association with the overall survival of patients. We demonstrated that ectopic overexpression of CASC11 significantly increased the proliferation, colony formation, and migration capacity in all three cell lines. CASC11 overexpression caused suppression of miR-145 and overexpression of IGF1R, leading to activation of PI3K/AKT/mTOR signaling pathway.
Conclusion
In summary, we found that CASC11 is upregulated in PCa cells and clinical tumor samples in comparison to corresponding controls and revealed that ectopic CASC11 overexpression promotes cellular phenotypes associated with PCa progression through CASC11/miR-145/IGF1R axis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ma Y, Fan B, Ren Z, Liu B, Wang Y. Long noncoding RNA DANCR contributes to docetaxel resistance in prostate cancer through targeting the miR-34a-5p/JAG1 pathway. Onco Targets Ther. 2019;12:5485–97.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
Janiczek M, Szylberg Ł, Kasperska A, Kowalewski A, Parol M, Antosik P, et al. Immunotherapy as a promising treatment for prostate cancer: a systematic review. J Immunol Res. 2017;2017:4861570.
Tian C, Deng Y, Jin Y, Shi S, Bi H. Long non-coding RNA RNCR3 promotes prostate cancer progression through targeting miR-185-5p. Am J Transl Res. 2018;10:1562–70.
Xu YH, Deng JL, Wang G, Zhu YS. Long non-coding RNAs in prostate cancer: Functional roles and clinical implications. Cancer Lett. 2019;464:37–55.
Maruyama R, Suzuki H. Long noncoding RNA involvement in cancer. BMB Rep. 2012;45:604–11.
Chen X, Sun Y, Cai R, Wang G, Shu X, Pang W. Long noncoding RNA: multiple players in gene expression. BMB Rep. 2018;51:280–9.
Yu H, Zhou W, Yan W, Xu Z, Xie Y, Zhang P. LncRNA CASC11 is upregulated in postmenopausal osteoporosis and is correlated with TNF-α. Clin Inter Aging. 2019;14:1663–9.
Hsu W, Liu L, Chen X, Zhang Y, Zhu W. LncRNA CASC11 promotes the cervical cancer progression by activating Wnt/beta-catenin signaling pathway. Biol Res. 2019;52:33.
Denaro N, Merlano MC, Lo Nigro C. Long noncoding RNAs as regulators of cancer immunity. Mol Oncol. 2019;13:61–73.
Misawa A, Takayama KI, Inoue S. Long non-coding RNAs and prostate cancer. Cancer Sci. 2017;108:2107–14.
Zhang Z, Zhou C, Chang Y, Hu Y, Zhang F, Lu Y, et al. Long non-coding RNA CASC11 interacts with hnRNP-K and activates the WNT/β-catenin pathway to promote growth and metastasis in colorectal cancer. Cancer Lett. 2016;376:62–73.
Han Y, Chen M, Wang A, Fan X. STAT3-induced upregulation of lncRNA CASC11 promotes the cell migration, invasion and epithelial-mesenchymal transition in hepatocellular carcinoma by epigenetically silencing PTEN and activating PI3K/AKT signaling pathway. Biochem Biophys Res Commun. 2019;508:472–9.
Zhang L, Kang W, Lu X, Ma S, Dong L, Zou B. LncRNA CASC11 promoted gastric cancer cell proliferation, migration and invasion in vitro by regulating cell cycle pathway. Cell Cycle. 2018;17:1886–1900.
Lin HY, Callan CY, Fang Z, Tung HY, Park JY. Interactions of. Cancer Epidemiol Biomark Prev. 2019;28:1067–75.
Kilic A, Barlak N, Sanli F, Aytatli A, Capik O, Karatas OF. Mode of action of carboplatin via activating p53/miR-145 axis in head and neck cancers. Laryngoscope. 2019;130:2818–24.
Ghorbanmehr N, Gharbi S, Korsching E, Tavallaei M, Einollahi B, Mowla SJ. miR-21-5p, miR-141-3p, and miR-205-5p levels in urine-promising biomarkers for the identification of prostate and bladder cancer. Prostate. 2019;79:88–95.
Hasegawa T, Glavich GJ, Pahuski M, Short A, Semmes OJ, Yang L, et al. Characterization and evidence of the miR-888 cluster as a novel cancer network in prostate. Mol Cancer Res. 2018;16:669–81.
Tinay I, Tan M, Gui B, Werner L, Kibel AS, Jia L. Functional roles and potential clinical application of miRNA-345-5p in prostate cancer. Prostate. 2018;78:927–37.
Wang L, Tang H, Thayanithy V, Subramanian S, Oberg AL, Cunningham JM, et al. Gene networks and microRNAs implicated in aggressive prostate cancer. Cancer Res. 2009;69:9490–7.
Ekin A, Karatas OF, Culha M, Ozen M. Designing a gold nanoparticle-based nanocarrier for microRNA transfection into the prostate and breast cancer cells. J Gene Med. 2014;16:331–5.
Karatas OF, Yuceturk B, Suer I, Yilmaz M, Cansiz H, Solak M, et al. Role of miR-145 in human laryngeal squamous cell carcinoma. Head Neck. 2016;38:260–6.
Seven M, Karatas OF, Duz MB, Ozen M. The role of miRNAs in cancer: from pathogenesis to therapeutic implications. Future Oncol. 2014;10:1027–48.
Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47:W556–60.
Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–58.
Ghandi M, Huang FW, Jané-Valbuena J, Kryukov GV, Lo CC, McDonald ER, et al. Next-generation characterization of the cancer cell line encyclopedia. Nature. 2019;569:503–8.
Mann M, Wright PR, Backofen R. IntaRNA 2.0: enhanced and customizable prediction of RNA-RNA interactions. Nucleic Acids Res. 2017;45:W435–9.
Ye Y, Yuan XH, Wang JJ, Wang YC, Li SL. The diagnostic value of miRNA-141 in prostate cancer: a systematic review and PRISMA-compliant meta-analysis. Medicine (Baltimore). 2020;99:e19993.
Örs Kumoğlu G, Döşkaya M, Gulce Iz S. The biomarker features of miR-145-3p determined via meta-analysis validated by qRT-PCR in metastatic cancer cell lines. Gene. 2019;710:341–53.
Zhou H, Zhu X. MicroRNA-21 and microRNA-30c as diagnostic biomarkers for prostate cancer: a meta-analysis. Cancer Manag Res. 2019;11:2039–50.
He S, Shi J, Mao J, Luo X, Liu W, Liu R, et al. The expression of miR-375 in prostate cancer: A study based on GEO, TCGA data and bioinformatics analysis. Pathol Res Pr. 2019;215:152375.
Xie ZC, Huang JC, Zhang LJ, Gan BL, Wen DY, Chen G, et al. Exploration of the diagnostic value and molecular mechanism of miR‑1 in prostate cancer: a study based on meta‑analyses and bioinformatics. Mol Med Rep. 2018;18:5630–46.
Yan HB, Zhang Y, Cen JM, Wang X, Gan BL, Huang JC, et al. Expression of microRNA-99a-3p in prostate cancer based on bioinformatics data and meta-analysis of a literature review of 965 cases. Med Sci Monit. 2018;24:4807–22.
Li D, Hao X, Song Y. Identification of the key MicroRNAs and the miRNA-mRNA regulatory pathways in prostate cancer by bioinformatics methods. Biomed Res Int. 2018;2018:6204128.
Khorasani M, Shahbazi S, Hosseinkhan N, Mahdian R. Analysis of differential expression of microRNAs and their target genes in prostate cancer: a bioinformatics study on microarray gene expression data. Int J Mol Cell Med. 2019;8:103–14.
Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol. 2013;11:14–23.
Hua JT, Chen S, He HH. Landscape of noncoding RNA in prostate cancer. Trends Genet. 2019;35:840–51.
Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47:199–208.
Cui Y, Shen G, Zhou D, Wu F. CASC11 overexpression predicts poor prognosis and regulates cell proliferation and apoptosis in ovarian carcinoma. Cancer Manag Res. 2020;12:523–9.
Yan R, Jiang Y, Lai B, Lin Y, Wen J. The positive feedback loop FOXO3/CASC11/miR-498 promotes the tumorigenesis of non-small cell lung cancer. Biochem Biophys Res Commun. 2019;519:518–24.
Luo H, Xu C, Le W, Ge B, Wang T. lncRNA CASC11 promotes cancer cell proliferation in bladder cancer through miRNA-150. J Cell Biochem. 2019;120:13487–93.
Chen J, Dang J. LncRNA CASC11 was downregulated in coronary artery disease and inhibits transforming growth factor. J Int Med Res. 2020;48:300060519889187.
Liang F, Yue J, Wang J, Zhang L, Fan R, Zhang H, et al. GPCR48/LGR4 promotes tumorigenesis of prostate cancer via PI3K/Akt signaling pathway. Med Oncol. 2015;32:49.
Wang H, Fang R, Wang XF, Zhang F, Chen DY, Zhou B, et al. Stabilization of Snail through AKT/GSK-3β signaling pathway is required for TNF-α-induced epithelial-mesenchymal transition in prostate cancer PC3 cells. Eur J Pharm. 2013;714:48–55.
Chen X, Yu Q, Pan H, Li P, Wang X, Fu S. Overexpression of IGFBP5 enhances radiosensitivity through PI3K-AKT pathway in prostate cancer. Cancer Manag Res. 2020;12:5409–18.
Wei A, Fan B, Zhao Y, Zhang H, Wang L, Yu X, et al. ST6Gal-I overexpression facilitates prostate cancer progression via the PI3K/Akt/GSK-3β/β-catenin signaling pathway. Oncotarget. 2016;7:65374–88.
Liao X, Thrasher JB, Holzbeierlein J, Stanley S, Li B. Glycogen synthase kinase-3beta activity is required for androgen-stimulated gene expression in prostate cancer. Endocrinology. 2004;145:2941–9.
Shorning BY, Dass MS, Smalley MJ, Pearson HB. The PI3K-AKT-mTOR pathway and prostate cancer: at the crossroads of AR, MAPK, and WNT signaling. Int J Mol Sci. 2020;21:4507.
Toren P, Zoubeidi A. Targeting the PI3K/Akt pathway in prostate cancer: challenges and opportunities (review). Int J Oncol. 2014;45:1793–801.
Heidegger I, Kern J, Ofer P, Klocker H, Massoner P. Oncogenic functions of IGF1R and INSR in prostate cancer include enhanced tumor growth, cell migration and angiogenesis. Oncotarget. 2014;5:2723–35.
Su J, Liang H, Yao W, Wang N, Zhang S, Yan X, et al. MiR-143 and MiR-145 regulate IGF1R to suppress cell proliferation in colorectal cancer. PLoS ONE. 2014;9:e114420.
Frasca F, Pandini G, Sciacca L, Pezzino V, Squatrito S, Belfiore A, et al. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem. 2008;114:23–37.
Vanamala J, Reddivari L, Radhakrishnan S, Tarver C. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer. 2010;10:238.
Zhu Z, Xu T, Wang L, Wang X, Zhong S, Xu C, et al. MicroRNA-145 directly targets the insulin-like growth factor receptor I in human bladder cancer cells. FEBS Lett. 2014;588:3180–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
OC, FS, AK, OC, IS, MK, and MI declare that they have no conflict of interests. OFK holds stocks in EcoTech Biotechnology. The terms of this arrangement have been reviewed and approved by Erzurum Technical University in accordance with its policy on objectivity in research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Capik, O., Sanli, F., Kurt, A. et al. CASC11 promotes aggressiveness of prostate cancer cells through miR-145/IGF1R axis. Prostate Cancer Prostatic Dis 24, 891–902 (2021). https://doi.org/10.1038/s41391-021-00353-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-021-00353-0