Abstract
Background:
Hundreds of ongoing clinical trials combine radiation therapy, mostly delivered as stereotactic body radiotherapy (SBRT), with immune checkpoint blockade. However, our understanding of the effect of radiotherapy on the intratumoral immune balance is inadequate, hindering the optimal design of trials that combine radiation therapy with immunotherapy. Our objective was to characterize the intratumoral immune balance of the malignant prostate after SBRT in patients.
Methods:
Sixteen patients with high-risk, non-metastatic prostate cancer at comparable Gleason Grade disease underwent radical prostatectomy with (n = 9) or without (n = 7) neoadjuvant SBRT delivered in three fractions of 8 Gy over 5 days completed 2 weeks before surgery. Freshly resected prostate specimens were processed to obtain single-cell suspensions, and immune-phenotyped for major lymphoid and myeloid cell subsets by staining with two separate 14-antibody panels and multicolor flow cytometry analysis.
Results:
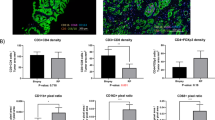
Malignant prostates 2 weeks after SBRT had an immune infiltrate dominated by myeloid cells, whereas malignant prostates without preoperative treatment were more lymphoid-biased (myeloid CD45+ cells 48.4 ± 19.7% vs. 25.4 ± 7.0%; adjusted p-value = 0.11; and CD45+ lymphocytes 51.6 ± 19.7% vs. 74.5 ± 7.0%; p = 0.11; CD3+ T cells 35.2 ± 23.8% vs. 60.9 ± 9.7%; p = 0.12; mean ± SD).
Conclusion:
SBRT drives a significant lymphoid to myeloid shift in the prostate-tumor immune infiltrate. This may be of interest when combining SBRT with immunotherapies, particularly in prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9:193–9.
Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2012;2:153.
Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83:1306–10.
Frey B, Rückert M, Weber J, Mayr X, Derer A, Lotter M, et al. Hypofractionated irradiation has immune stimulatory potential and induces a timely restricted infiltration of immune cells in colon cancer tumors. Front Immunol. 2017;8:231–231.
Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618.
Lee Y, Auh SL, Wang Y, Burnette B, Meng Y, Beckett M, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95.
Filatenkov A, Baker J, Mueller AM, Kenkel J, Ahn GO, Dutt S, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res. 2015;21:3727–39.
Schaue D. A Century of Radiation Therapy and Adaptive Immunity. Front Immunol. 2017;8:431. https://doi.org/10.3389/fimmu.2017.00431.
Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. 2012;12:191–200.
Formenti SC, Lee P, Adams S, Goldberg JD, Li X, Xie MW, et al. Focal irradiation and systemic tgfbeta blockade in metastatic breast cancer. Clin Cancer Res. 2018;24:2493–504.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol). 1995;57:289–300.
Capanu M, Seshan VE. False discovery rates for rare variants from sequenced data. Genet Epidemiol. 2015;39:65–76.
Amar D, Shamir R, Yekutieli D. Extracting replicable associations across multiple studies: Empirical Bayes algorithms for controlling the false discovery rate. PLOS Computational Biol. 2017;13:e1005700.
Efron B. Size, power and false discovery rates. Ann Stat. 2007;35:1351–77.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl J Med. 2012;366:2443–54.
Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–12.
Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35:40–7.
Abida W, Cheng ML, Armenia J, Middha S, Autio KA, Vargas HA, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5:471–8.
Wu YM, Cieslik M, Lonigro RJ, Vats P, Reimers MA, Cao X, et al. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell. 2018;173:1770–.e1714.
Ribas A, Shin DS, Zaretsky J, Frederiksen J, Cornish A, Avramis E, et al. PD-1 blockade expands intratumoral memory T cells. Cancer Immunol Res. 2016;4:194–203.
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4.
Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120:16–25.
Weber R, Fleming V, Hu X, Nagibin V, Groth C, Altevogt P, et al. Myeloid-derived suppressor cells hinder the anti-cancer activity of immune checkpoint inhibitors. Front Immunol. 2018;9:1310.
Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103.
Zhao SG, Lehrer J, Chang SL, Das R, Erho N, Liu Y, et al. The immune landscape of prostate cancer and nomination of PD-L2 as a potential therapeutic target. J Natl Cancer Inst. 2019;111:301–10.
Bercovici N, Guérin MV, Trautmann A, Donnadieu E. The Remarkable Plasticity of Macrophages: A Chance to Fight Cancer. Front Immunol. 2019;10:1563. https://doi.org/10.3389/fimmu.2019.01563.
DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro-versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309–16.
Theelen W, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts J, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1276–82.
Acknowledgements
We acknowledge NIH grants: 5R01CA191234-05 (DS), UCLA Prostate SPORE P50CA0912131 (RR, NN), Prostate Cancer Foundation Young Investigator Award (NN, AK). We would like to express our sincere thanks to all the patients and to the clinical research staff.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nickols, N.G., Ganapathy, E., Nguyen, C. et al. The intraprostatic immune environment after stereotactic body radiotherapy is dominated by myeloid cells. Prostate Cancer Prostatic Dis 24, 135–139 (2021). https://doi.org/10.1038/s41391-020-0249-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-020-0249-8
This article is cited by
-
Significant changes in macrophage and CD8 T cell densities in primary prostate tumors 2 weeks after SBRT
Prostate Cancer and Prostatic Diseases (2023)
-
Immunomodulatory effects of carbon ion radiotherapy in patients with localized prostate cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
PSA bounce, prognosis, and clues to the radiation response
Prostate Cancer and Prostatic Diseases (2021)