Abstract
Background
The relationship between metformin use and prostate cancer risk remains controversial. Genetic variation in metformin metabolism pathways appears to modify metformin glycemic control and the protective association with some cancers. However, no studies to date have examined this pharmacogenetic interaction and prostate cancer chemoprevention.
Methods
Clinical data and germline DNA were collected from our prostate biopsy database between 1996 and 2014. In addition to a genome-wide association study (GWAS), 27 single nucleotide polymorphisms (SNPs) implicated in metformin metabolism were included on a custom SNP array. Associations between metformin use and risk of high-grade (Grade Group ≥ 2) and overall prostate cancer were explored using a case-control design. Interaction between the candidate/GWAS SNPs and the metformin-cancer association was explored using a case-only design.
Results
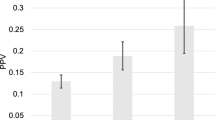
Among 3481 men, 132 (4%) were taking metformin at diagnosis. Metformin users were older, more likely non-Caucasian, and had higher body mass index, Gleason score, and number of positive cores. Overall, 2061 (59%) were diagnosed with prostate cancer, of which 922 (45%) were high-grade. After adjusting for baseline characteristics, metformin use was associated with higher risk of high-grade prostate cancer (OR = 1.76, 95% CI 1.1–2.9, p = 0.02) and overall prostate cancer (OR = 1.77, 95% CI 1.1–2.9, p = 0.03). None of the 27 candidate SNPs in metformin metabolic pathways had significant interaction with the metformin-cancer association. Among the GWAS SNPs, one SNP (rs149137006) had genome-wide significant interaction with metformin for high-grade prostate cancer, and another, rs115071742, for overall prostate cancer. They were intronic and intergenic SNPs, respectively, with largely uncharacterized roles in prostate cancer chemoprevention.
Conclusions
In our cohort, metformin use was associated with increased risk of being diagnosed with prostate cancer. While SNPs involved in metformin metabolism did not have modifying effects on the association with disease risk, one intronic and one intergenic SNP from the GWAS study did, and these require further study.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
American Cancer Society. Key statistics for prostate cancer. Atlanta, Georgia, United: American Cancer Society; 2019.
Carlson LE, Angen M, Cullum J, Goodey E, Koopmans J, Lamont L, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer. 2004;90:2297–304.
Balderson N, Towell T. The prevalence and predictors of psychological distress in men with prostate cancer who are seeking support. Br J Health Psychol. 2003;8(Pt 2):125–34.
Resnick MJ, Koyama T, Fan KH, Albertsen PC, Goodman M, Hamilton AS, et al. Long-term functional outcomes after treatment for localized prostate cancer. N. Engl J Med. 2013;368:436–45.
Saenz A, Fernandez-Esteban I, Mataix A, Ausejo M, Roque M, Moher D. Metformin monotherapy for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;3:CD002966.
Ben Sahra I, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P, et al. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010;70:2465–75.
Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–86.
Tsutsumi Y, Nomiyama T, Kawanami T, Hamaguchi Y, Terawaki Y, Tanaka T, et al. Combined treatment with Exendin-4 and metformin attenuates prostate cancer growth. PLoS ONE. 2015;10:e0139709.
Zaidi S, Gandhi J, Joshi G, Smith NL, Khan SA. The anticancer potential of metformin on prostate cancer. Prostate Cancer Prostatic Dis. 2019;3:351–61.
Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Antidiabetic medication and prostate cancer risk: a population-based case-control study. Am J Epidemiol. 2008;168:925–31.
Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control. 2009;20:1617–22.
Ruiter R, Visser LE, van Herk-Sukel MP, Coebergh JW, Haak HR, Geelhoed-Duijvestijn PH, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care. 2012;35:119–24.
Preston MA, Riis AH, Ehrenstein V, Breau RH, Batista JL, Olumi AF, et al. Metformin use and prostate cancer risk. Eur Urol. 2014;66:1012–20.
Margel D, Urbach D, Lipscombe LL, Bell CM, Kulkarni G, Austin PC, et al. Association between metformin use and risk of prostate cancer and its grade. J Natl Cancer Inst. 2013;105:1123–31.
Azoulay L, Dell’Aniello S, Gagnon B, Pollak M, Suissa S. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomark Prev. 2011;20:337–44.
Feng T, Sun X, Howard LE, Vidal AC, Gaines AR, Moreira DM, et al. Metformin use and risk of prostate cancer: results from the REDUCE study. Cancer Prev Res. 2015;8:1055–60.
Wang DS, Jonker JW, Kato Y, Kusuhara H, Schinkel AH, Sugiyama Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J Pharmacol Exp Ther. 2002;302:510–5.
Shu Y, Brown C, Castro RA, Shi RJ, Lin ET, Owen RP, et al. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharm Ther. 2008;83:273–80.
Zhou K, Donnelly LA, Kimber CH, Donnan PT, Doney AS, Leese G, et al. Reduced-function SLC22A1 polymorphisms encoding organic cation transporter 1 and glycemic response to metformin: a GoDARTS study. Diabetes. 2009;58:1434–9.
Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH. Genetic variation in the multidrug and toxin extrusion 1 transporter protein influences the glucose-lowering effect of metformin in patients with diabetes: a preliminary study. Diabetes. 2009;58:745–9.
Stocker SL, Morrissey KM, Yee SW, Castro RA, Xu L, Dahlin A, et al. The effect of novel promoter variants in MATE1 and MATE2 on the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacol Ther. 2013;93:186–94.
Choi JH, Yee SW, Ramirez AH, Morrissey KM, Jang GH, Joski PJ, et al. A common 5’-UTR variant in MATE2-K is associated with poor response to metformin. Clin Pharmacol Ther. 2011;90:674–84.
Dong M, Gong ZC, Dai XP, Lei GH, Lu HB, Fan L, et al. Serine racemase rs391300 G/A polymorphism influences the therapeutic efficacy of metformin in Chinese patients with diabetes mellitus type 2. Clin Exp Pharmacol Physiol. 2011;38:824–9.
GoDarts, Group UDPS, Wellcome Trust Case Control Consortium, Zhou K, Bellenguez C, Spencer CC, et al. Common variants near ATM are associated with glycemic response to metformin in type 2 diabetes. Nat Genet. 2011;43:117–20.
Pacanowski MA, Hopley CW, Aquilante CL. Interindividual variability in oral antidiabetic drug disposition and response: the role of drug transporter polymorphisms. Expert Opin Drug Metab Toxicol. 2008;4:529–44.
Clarke GM, Morris AP. A comparison of sample size and power in case-only association studies of gene-environment interaction. Am J Epidemiol. 2010;171:498–505.
Kote-Jarai Z, Olama AA, Giles GG, Severi G, Schleutker J, Weischer M, et al. Seven prostate cancer susceptibility loci identified by a multi-stage genome-wide association study. Nat Genet. 2011;43:785–91.
Kote-Jarai Z, Easton DF, Stanford JL, Ostrander EA, Schleutker J, Ingles SA, et al. Multiple novel prostate cancer predisposition loci confirmed by an international study: the PRACTICAL Consortium. Cancer Epidemiol Biomark Prev. 2008;17:2052–61.
Akinyeke T, Matsumura S, Wang X, Wu Y, Schalfer ED, Saxena A, et al. Metformin targets c-MYC oncogene to prevent prostate cancer. Carcinogenesis. 2013;34:2823–32.
Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011;9:33.
Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996;81:4059–67.
Frasca F, Pandini G, Sciacca L, Pezzino V, Squatrito S, Belfiore A, et al. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem. 2008;114:23–37.
Lima GA, Correa LL, Gabrich R, Miranda LC, Gadelha MR. IGF-I, insulin and prostate cancer. Arq Bras Endocrinol Metab. 2009;53:969–75.
Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90.
Markman B, Atzori F, Perez-Garcia J, Tabernero J, Baselga J. Status of PI3K inhibition and biomarker development in cancer therapeutics. Ann Oncol. 2010;21:683–91.
Sarker D, Reid AH, Yap TA, de Bono JS. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res. 2009;15:4799–805.
Colquhoun AJ, Venier NA, Vandersluis AD, Besla R, Sugar LM, Kiss A, et al. Metformin enhances the antiproliferative and apoptotic effect of bicalutamide in prostate cancer. Prostate Cancer Prostatic Dis. 2012;15:346–52.
Wu GF, Zhang XL, Luo ZG, Yan JJ, Pan SH, Ying XR, et al. Metformin therapy and prostate cancer risk: a meta-analysis of observational studies. Int J Clin Exp Med. 2015;8:13089–98.
Suissa S, Azoulay L. Metformin and cancer: mounting evidence against an association. Diabetes Care. 2014;37:1786–8.
Segawa T, Nau ME, Xu LL, Chilukuri RN, Makarem M, Zhang W, et al. Androgen-induced expression of endoplasmic reticulum (ER) stress response genes in prostate cancer cells. Oncogene. 2002;21:8749–58.
Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29:452–63.
Wang D, Ding L, Wang L, Zhao Y, Sun Z, Karnes RJ, et al. LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer. Oncotarget. 2015;6:41045–55.
Marques Howarth M, Simpson D, Ngok SP, Nieves B, Chen R, Siprashvili Z, et al. Long noncoding RNA EWSAT1-mediated gene repression facilitates Ewing sarcoma oncogenesis. J Clin Invest. 2014;124:5275–90.
Agrelo R, Souabni A, Novatchkova M, Haslinger C, Leeb M, Komnenovic V, et al. SATB1 defines the developmental context for gene silencing by Xist in lymphoma and embryonic cells. Dev Cell. 2009;16:507–16.
Ling H, Spizzo R, Atlasi Y, Nicoloso M, Shimizu M, Redis RS, et al. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013;23:1446–61.
Wang Y, Wang Z, Xu J, Li J, Li S, Zhang M, et al. Systematic identification of non-coding pharmacogenomic landscape in cancer. Nat Commun. 2018;9:3192.
Parasramka M, Yan IK, Wang X, Nguyen P, Matsuda A, Maji S, et al. BAP1 dependent expression of long non-coding RNA NEAT-1 contributes to sensitivity to gemcitabine in cholangiocarcinoma. Mol Cancer. 2017;16:22.
Prensner JR, Chen W, Iyer MK, Cao Q, Ma T, Han S, et al. PCAT-1, a long noncoding RNA, regulates BRCA2 and controls homologous recombination in cancer. Cancer Res. 2014;74:1651–60.
Bester AC, Lee JD, Chavez A, Lee YR, Nachmani D, Vora S, et al. An integrated genome-wide CRISPRa approach to functionalize lncRNAs in drug resistance. Cell. 2018;173:649–64 e620.
Acknowledgements
RJH funding from CCSRI (Grant#702108) and PCC (Grant#D2013-17) and CUASF Career Development for Research Grant 2017-2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lee, M.J., Jayalath, V.H., Xu, W. et al. Association between metformin medication, genetic variation and prostate cancer risk. Prostate Cancer Prostatic Dis 24, 96–105 (2021). https://doi.org/10.1038/s41391-020-0238-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-020-0238-y
This article is cited by
-
Targeting of the tumor immune microenvironment by metformin
Journal of Cell Communication and Signaling (2022)
-
Response to the Letter to the Editor: “Association between metformin medication, genetic variation and prostate cancer risk”—genotyping and patient categorization, do they matter?
Prostate Cancer and Prostatic Diseases (2021)
-
Letter to the Editor: “Association between metformin medication, genetic variation and prostate cancer risk”—genotyping and patient categorizations, do they matter?
Prostate Cancer and Prostatic Diseases (2021)