Abstract
Background
Previous study reported shared decision making was underused in PSA-based prostate cancer screening. In mid-2018, the US Preventive Service Task Force recommended shared decision making (SDM) before PSA-based prostate cancer screening among men aged 55–69 year while remained against PSA testing in men aged 70 or older. The objective of this study is to examine recent changes in SDM and prostate cancer screening following recent USPSTF recommendations.
Methods
A retrospective cross-sectional study among men aged 50 years or older were conducted using 2015 and 2018 National Health Interview Survey data (n = 10,926). Outcomes included self-reported PSA testing for prostate cancer screening last year, and if yes, whether respondent ever had a discussion with the healthcare provider about its advantages and disadvantages. Analyses were stratified by respondent’s age (50–54 vs. 55–69 vs. 70+).
Results
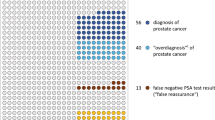
Routine PSA screening rates remained stable from 34.3% in 2015 to 35.4% in first half of 2018, and 36.0% in second half of 2018 (p trend = 0.57). A similar pattern was found in men ≥70 years (p trend = 0.98). Receipt of SDM increased in men aged ≥50 years from 30.5% in 2015 to 33.6% in first half of 2018, and 36.7% in second half of 2018 (p trend = 0.002). The increase was most prominent in men aged 55 to 69 years (31.6, 36.9, and 40.2% in 2015, first half of 2018 and second half of 2018 respectively; p trend = 0.001).
Conclusions
Between 2015 and 2018, there was no significant increase in the PSA-based prostate cancer screening. However, a significant increasing trend in SDM was observed, especially in men aged 55–69 years.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Wolf AM, Wender RC, Etzioni RB, Thompson IM, D’Amico AV, Volk RJ, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60:70–98.
Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190:419–26.
USPSTF. Screening for prostate cancer: US preventive services task force recommendation statement. JAMA. 2018;319:1901–13.
Leyva B, Persoskie A, Ottenbacher A, Hamilton JG, Allen JD, Kobrin SC, et al. Do men receive information required for shared decision making about psa testing? Results from a national survey. J Cancer Educ. 2016;31:693–701.
Fedewa SA, Gansler T, Smith R, Sauer AG, Wender R, Brawley OW, et al. Recent patterns in shared decision making for prostate-specific antigen testing in the United States. Ann Fam Med. 2018;16:139–44.
National Center for Health Statistics. National Health Interview Survey. Public-use data file and documentation (http://www.cdc.gov/nchs/nhis/nhis_questionnaires.htm). National Center for Health Statistics; 2018.
Li J, Ding H, Richards TB, Martin I, Kobrin S, Marcus PM. Prostate-specific antigen testing initiation and shared decision-making: Findings from the 2000 and 2015 national health interview surveys. J Am Board Fam Med. 2018;31:658–62.
Fedewa SA, Ward EM, Brawley O, Jemal A. Recent patterns of prostate-specific antigen testing for prostate cancer screening in the United States. JAMA Intern Med. 2017;177:1040–2.
Jemal A, Fedewa SA, Ma J, Siegel R, Lin CC, Brawley O, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314:2054–61.
Moyer VA. USPSTF. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–34.
Lansdorp-Vogelaar I, Gulati R, Mariotto AB, Schechter CB, De Carvalho TM, Knudsen AB, et al. Personalizing age of cancer screening cessation based on comorbid conditions: model estimates of harms and benefits. Ann Intern Med. 2014;161:104–12.
Heijnsdijk EAM, Wever EM, Auvinen A, Hugosson J, Ciatto S, Nelen V, et al. Quality-of-life effects of prostate-specific antigen screening. N Engl J Med. 2012;367:595–605.
Torke AM, Schwartz PH, Holtz LR, Montz K, Sachs GA. Older adults and forgoing cancer screening: “I Think It Would Be Strange”. JAMA Intern Med. 2013;173:526–31.
Acknowledgements
CJ and XH had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: All authors. Acquisition, analysis, or interpretation of data: All authors. Drafting of the paper: All authors. Critical revision of the paper for important intellectual content: All authors. Statistical analysis: CJ and XH. Administrative, technical, or material support: CJ and XH. Supervision: SAF, AJ, and XH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Jiang, C., Fedewa, S.A., Wen, Y. et al. Shared decision making and prostate-specific antigen based prostate cancer screening following the 2018 update of USPSTF screening guideline. Prostate Cancer Prostatic Dis 24, 77–80 (2021). https://doi.org/10.1038/s41391-020-0227-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-020-0227-1
This article is cited by
-
Shared decision-making before prostate cancer screening decisions
Nature Reviews Urology (2024)
-
Liquid biomarkers for early detection of prostate cancer and summary of available data for their use in African-American men
Prostate Cancer and Prostatic Diseases (2022)
-
Examination of prostate-specific antigen (PSA) screening in military and civilian men: analysis of the 2018 behavioral risk factor surveillance system
Cancer Causes & Control (2022)
-
Improvement of quality of life and symptom burden after robot-assisted radical prostatectomy in patients with moderate to severe LUTS
Scientific Reports (2021)