Abstract
Background
Androgen-targeted therapy and chemotherapy are currently the mainstay of treatment in metastatic castration resistant prostate cancer (mCRPC). When progression occurs despite these therapeutic strategies, additional FDA-approved treatment options are lacking. However, there is a vast amount of emerging data surrounding novel investigational therapies in this space.
Methods
We reviewed and summarized the body of literature surrounding the current treatment options for mCRPC. Medline and Pubmed as well as abstracts from international congresses were utilized to gather relevant literature surrounding investigational treatment of mCRPC. We highlight the results of recent trials investigating the use of novel strategies to treat mCRPC.
Results
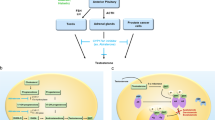
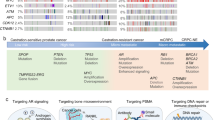
Androgen-targeted therapy and chemotherapy will remain foundational in the treatment of mCRPC. However, heavily pretreated patients who have developed resistance may benefit from novel therapeutic strategies. The use of poly(adenosine diphosphate [ADP]-ribose) polymerase inhibitors (PARPi) has now gained FDA approval for patients with homologous recombination repair (HRR) gene mutations. Novel androgen receptor (AR) degraders and the use of radioligand therapy (RLT) with Lu-PSMA-617 (Lu-PSMA) are under investigation. Immune-directed therapies, including programmed death (PD-1) inhibition, bi-specific T-cell engager (BiTE) technology, and chimeric antigen receptor (CAR) T-cell therapy, have shown promise in early phase trials. Further understanding of resistance mechanisms has led to additional therapeutic targets, including targeting the PI3K-Akt-mTOR pathway and enhancer of zester homolog 2 (EZH2).
Conclusions
Based on our review of the literature, exciting new therapeutic strategies exist for the treatment of mCRPC. In particular, PARPi, AR degraders, PSMA-targeted therapies, immune-directed therapies, and agents targeting resistance mechanisms as monotherapy or in combination could improve patient outcomes. Additional data from randomized trials are necessary to understand the efficacy and tolerability of these treatment strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. Ca-a Cancer J Clin. 2018;68:7–30.
Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pr. 2011;65:1180–92.
Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–18.
James ND, Spears MR, Clarke NW, Dearnaley DP, De Bono JS, Gale J, et al. Survival with newly diagnosed metastatic prostate cancer in the “docetaxel era”: data from 917 patients in the control arm of the STAMPEDE trial. Eur Urol. 2105;67:1028–38.
Halabi S, Kelly WK, Ma H, Zhou H, Solomon NC, Fizazi K, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in med with castration-resistant prostate cancer. J Clin Oncol. 2016;34:1652–9.
He L, Fang H, Chen C, Wu Y, et al. Metastatic castration-resistant prostate cancer: academic insights and perspectives through bibliometric analysis. Medicine. 2020;99:e19760.
Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl J Med. 2004;351:1513–20.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl J Med. 2004;351:1502–1.
Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders FA, Sternborg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naïve men with metastatic castration resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomized, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–60.
De Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl J Med. 2011;364:1995–2005.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl J Med. 2012;367:1187–97.
Beer TM, Armstrong AJ, Rathkopf D, Lorit Y, Sternberg CN, Higano CS, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151–4.
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration resistant prostate cancer. N. Engl J Med. 2010;363:411–22.
Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alphe emitter radium-223 and survival in metastatic prostate cancer. N. Engl J Med. 2013;369:213–23.
Tsao CK, Cutting E, Martin J, Oh WK. The role of cabazitaxel in the treatment of metastatic castration-resistant prostate cancer. Ther Adv Urol. 2014;6:97–104.
Attard G, Borre M, Gurney H, et al. Abiraterone alone or in combination with enzalutamide in mestatatic castration-resistant prostate cancer with rising prostate-specific antigen during enzalutamide treatment. J Clin Oncol. 2018;36:2639–46.
Khalaf DJ, Annala M, Taavitsainen S, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in mestatatic castration-resistant prostate cancer: a multicenter, randomized, open-label, phase 2, crossover trial. Lancet Oncol. 2019;20:1730–9.
Gillessen S, Attard G, Beer T, et al. Management of pateints with advanced prostate cancer: the report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol. 2018;73:178–211.
De Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N. Engl J Med. 2019;381:2506–18.
Adashek JJ, Jain, RK, Zhang J. Clinical development of PARP inhibitors in treating metastatic castration-resistant prostate cancer. Cells. 2019;8:860.
Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-repair defects and Olaparib in metastatic prostate cancer. N. Engl J Med. 2015;373:1697–708.
Mateo J, Porta N, Bianchini D, McGovern U, Elliott T, Jones R, et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21:162–74.
de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl J Med. 2020;382:2091–102.
De Bono, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Final overall survival analysis of PROfound: Olaparib vs physician’s choice of enzalutamide or abiraterone in patients with metastatic castration-resistant prostate cancer and homologous recombination repair gene alterations. Ann Oncol. 2020;31(suppl_4):S507–49.
Abida W, Campbell D, Patnaik A, Shapiro JD, Sautois B, Vogelzang NJ, et al. Preliminary results from TRITON2: a phase 2 study of rucaparib in patients with Metastatic Castration-Resistant Prostate Cancer (mCRPC) associated with homologous recombination repair (HRR) gene alternations: updated analyses. Ann Onc. 2019;30:327–8.
Abida W, Campbell D, Patnaik A, Shapiro JD, Sautois B, Vogelzang NJ, et al. Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: analysis from the Phase II TRITON2 Study. Clin Cancer Res. 2020;26:2487–96.
Clarke N, Wiechno P, Alekseev B, Sala N, Jones R, Kocak I, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19:975–86.
ClinicalTrials.gov. National Library of Medicine (U.S.). (2000, February 29–). Study on Olaparib Plus Abiraterone as First-line Therapy in Men With Metastatic Castration-Resistant Prostate Cancer. Identifier: NCT03732820. Retrieved August 4, 2020 from https://clinicaltrials.gov/ct2/show/NCT03732820.
ClinicalTrials.gov. National Library of Medicine (U.S.). (2000, February 29–). TRAP: Targeting Resistant Prostate Cancer With ATR and PARP Inhibition. Identifier: NCT03787680. Retrieved August 4, 2020 from https://clinicaltrials.gov/ct2/show/NCT03787680.
ClinicalTrials.gov. National Library of Medicine (U.S.). (2000, February 29–). Testosterone and Olaparib in Treating Patients With Castration-Resistant Prostate Cancer. Identifier: NCT03516812. Retrieved August 4, 2020 from https://clinicaltrials.gov/ct2/show/NCT03516812.
Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharm. 2017;8:561.
Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol. 2020;38:395–405.
Shaffer DR JW, Massard C, et al. A phase Ia study of safety and clinical activity of atezolizumab (atezo) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2018;36:187–187.
Le DT, Uram NJ, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch repair deficiency. N. Engl J Med. 2015;372:2509–20.
Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: results from the phase II KEYNOTE-158 study. Am J Clin Oncol. 2020;38:1–10.
Yu E, Piulats JM, Gravis G, Laguerre B, Arija JA, Oudard S, et al. KEYNOTE-365 cohort A updated results: Pembrolizumab (pembro) plus olaparib in docetaxel-pretreated patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2020;38:100.
Massard C, Retz M, Hammerer P, Quevedo F, Fong PC, Berry WR, et al. Keynote-365 cohort b: pembrolizumab (pembro) plus docetaxel and prednisone in abiraterone (abi) or enzalutamide (enza)-pretreated patients (pts) with metastatic castrate resistant prostate cancer (mCRPC). J Clin Oncol. 2019;37:170.
Fong PCC, Retz M, Drakaki A, Massard C, Berry WR, Romano E, et al. Keynote-365 cohort C: pembrolizumab (pembro) plus enzalutamide (enza) in abiraterone (abi)-pretreated patients (pts) with metastatic castrate resistant prostate cancer (mCRPC). J Clin Oncol. 2019;37:171.
Graff J. KEYNOTE-199: pembrolizumab plus enzalutamide for enzalutamide-resistant mCRPC: cohorts 4-5. J Clin Oncol. 2020;38(6_suppl):15.
Graff JN, Beer TM, Alumkal JJ, Slottke RE, Redmond WL, Thomas GV, et al. A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone. J Immunother Cancer. 2020;8:e000642.
Sweeney CJ, Gillessen S, Rathkopf D, et al. IMbassador250: A phase III trial comparing atezolizumab with enzalutamide vs enzalutamide alone in patients with metastatic castration-resistant prostate cancer (mCRPC). Presented at: American Association for Cancer Research (AACR) Virtual Annual Meeting I 2020; April 27–28, 2020. Abstract CT014.
Beretta GL, Zaffaroni N. Androgen receptor-directed molecular conjugates for targeting prostate cancer. Front Chem. 2019;7:369.
Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15:701–11.
Narayanan R, Ponnusamy S, Miller DD. Destroying the androgen receptor (AR)-potential strategy to treat advanced prostate cancer. Oncoscience. 2017;4:175–77.
Bradbury RH, Hales NJ, Rabow AA, Walker GE, Acton DG, et al. Small-molecule androgen receptor downregulators as an approach to treatment of advanced prostate cancer. Bioorg Med Chem Lett. 2011;21:5442–5.
Omlin A, Jones RJ, van der Noll R, Satoh T, Niwakawa M, Smith SA, et al. AZD3514, an oral selective androgen receptor down-regular in patient with castration-resistant prostate cancer – results of two parallel first-in-human phase I studies. Investig N. Drugs. 2015;33:679–90.
Wang R, Sun Y, Li L, Niu Y, Lin W, Lin C, et al. Preclinical study using Malat1 small interfering RNA or andreogen receptor splicing variant 7 degradation enhancer ASC-J9 to suppress enzalutamide-resistant prostate cancer progression. Eur Urol. 2017;72:835–44.
Wang R, Lin W, Lin C, Li L, Sun Y, Change C. ASC-J9 suppresses castration resistant prostate cancer progression via degrading the enzalutamide induced androgen receptor mutant AR-F876L. Cancer Lett. 2016;379:154–60.
Luo J, Tian J, Chou F, Lin C, Xing EZ, Zuo L, et al. Targeting the androgen receptor (AR) with AR degradation enhancer ASC-J9 led to increase docetaxel sensitivity via suppressing the p21 expression. Cancer Lett. 2019;444:35–44.
Gustafson JL, Neklesa TK, Cox CS, Roth AG, Buckley DL, Tae HS, et al. Small molecule mediated degradation of the androgen receptor through hydrophobic tagging. Angew Chem Int Ed Engl. 2015;54:9659–62.
Han X, Wang C, Qin C, Xiang W, Fernandez-Salas E, Yang CY, et al. Discovery of ARD-69 as a highly potent proteolysis targeting chimera (PROTAC) degrader of androgen receptor (AR) for the treatment of prostate cancer. J Med Chem. 2019;62:941–64.
Neklesa TK, Snyder LB, Bookbinder M, Chen X, Crew AP, Crews CM, et al. An oral androgen receptor PROTAC degrader for prostate cancer. J Clin Oncol. 2017;35:273.
Petrylak DP, Gao X, Vogelzang NJ, Garfield MH, Taylor I, Moore MD, et al. First-in-human phase I study of ARV-110, an androgen receptor PROTAC degrader in patients with metastatic castrate-resistant prostate cancer following enzalutamide and/or abiraterone. J Clin Oncol. 2020;38(15_suppl):3500.
Novakova Z, Foss CA, Copeland BT, Morath V, Baranova P, Havlinova B, et al. Novel monoclonal antibodies recognizing human prostate specific membrane antigen (PSMA) as research and theranostic tools. Prostate. 2017;77:749–64.
Ahmadzadehfar H, Rahbar K, Kurpig S, Bogemann M, Claesener M, Eppard E. et al. Early side effects and first results of radioligand therapy with (9177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 2015;5:114.
Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177 Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33.
Hofman MS, Emmett L, Sandhu SK, Iravani A, Joshua AM, Goh JC, et al. TheraP: a randomized phase II trial of 177Lu-PSMA-617 theranostic versus cabazitaxel in metastatic castration resistant prostate cancer progressing after docetaxel: initial results (ANZUP protocol 1603). J Clin Oncol. 2020;38(15_suppl):5500.
ClinicalTrials.gov. National Library of Medicine (U.S.). (2000, February 29–). Study of 177Lu-PSMA-617 in metastatic castrate resistant prostate cancer (VISION). Identifier NCT03511664. Retrieved August 4, 2020. from https://clinicaltrials.gov/ct2/show/NCT03511664.
Einsele H, Borghaei H, Orlowski RZ, Subklewe M, Roboz GJ, Zugmaier G, et al. The BiTE (Bispecific T-cell engager) platform: development and future potential of a targeted immune-oncology therapy across tumor types. Cancer. 2020;126:3192–201.
Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl J Med. 2017;376:836–47.
Hummel HD, Kufer P, Grullich C, et al. Phase 1 study of pasotuxizumab (BAY 2010112), a PSMA-targeting bispecific T cell engager (BiTE) immunotherapy for metastatic castration-resistant prostate cancer (mCRPC) [abstract]. J Clin Oncol. 2019;37:5034.
Bailis J, Deegen P, Thomas O, Bogner P, Wahl J, Liao M, et al. Preclinical evaluation of AMG 160, a next generation bispecfic T cell engager (BiTE) targeting the prostate-specific membrane antigen PSMA for metastatic castration-resistant prostate cancer (mCRPC) [abstract]. J Clin Oncol. 2019;37:301.
Tran B, Horvath L, Dorff T, et al. Results from a phase I study of AMG 160, a half-life extended, PSMA-tareted, bispecific T cell engager immune therapy for metastatic castration-resistant prostate cancer. Ann Oncol. 2020;31(suppl_4):S507–49.
Yu H, Pan J, Guo Z, Yang C, Mao L. CART cell therapy for prostate cancer: status and promise. Onco Targets Ther. 2019;12:391–5.
Holstein SA, Lunning MA. CAR T-cell therapy in hematologic malignancies: a voyage in progress. Clin Pharm Ther. 2020;107:112–22.
Schepisi G, Cursano MC, Casadei C, Menna C, Altavilla A, Lolli C, et al. CAR-T cell therapy: a potential new strategy against prostate cancer. J Immunother Cancer. 2019;7:258.
Ma Q, Gomes EM, Lo AS, Junghans RP. Advanced generation anti-prostate specific membrane antigen designer T cells for prostate cancer immunotherapy. Prostate. 2014;74:286–96.
Slovin SF, Wang X, Hullings M, Arauz G, Bartido S, Lewis JS, et al. Chimeric antigen receptor (CAR+) modified T cells targeting prostate-specific membrane antigen (PSMA) in patients (pts) with castrate metastatic prostate cancer (CMPC). J Clin Oncol. 2013;31:72.
Junghans RP, Qiangzhong M, Rathore R, Gomes EM, Bais AJ, Lo AS, et al. Phase I trial of Anti-PSMA designer CAR-T cells in prostate cancer: possible role for interacting interleukin 2-T cell pharmacodynamics as a determinant of clinical response. Prostate. 2016;76:1257–70.
Kloss CC, Lee J, Zhang A, Chen F, Melenhorst JJ, Lacey SF, et al. Dominant-negative TGF-β receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol Ther. 2018;26:1855–66.
Narayan V, Gladney W, Plesa G, Vapiwala N, Carpenter E, Maude SL, et al. A phase I clinical trial of PSMA-directed/TGFβ-insensitive CAR-T cells in metastatic castration-resistant prostate cancer. J Clin Oncol. 2019;37(7_suppl).
John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19:5636–46.
Sanchez C, Chan R, Bajgain P, Rambally S, Palapattu G, Mims M, et al. Combining T-cell immunotherapy and anti-androgen therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2013;16:123–31.
Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–39.
Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–86.
Mulholland DJ, Tran LM, Li Y, Cai H, Morim A, Wang S, et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. 2011;19:792–804.
Ferraldeschi R, NavaRodrigues D, Riisnaes R, Miranda S, Figueiredo I, Rescigno P, et al. PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur Urol. 2015;67:795–802.
Wise-Draper TM, Moorthy G, Salkeni MA, Karim NA, Thomas HE, Mercer CA, et al. A Phase Ib Study of the Dual PI3K/mTOR Inhibitor Dactolisib (BEZ235) combined with everolimus in patients with advanced solid malignancies. Target Oncol. 2017;12:323–32.
Dolly SO, Wagner AJ, Bendell JC, Kindler HL, Krug LM, Seiwert TY, et al. Phase I Study of Apitolisib (GDC-0980), dual phosphatidylinositol-3-kinase and mammalian target of rapamycin kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2016;22:2874–84.
ClinicalTrials.gov. National Library of Medicine (U.S.). (2000, February 29–). Study of Ipatasertib or Apitolisib With Abiraterone Acetate Versus Abiraterone Acetate in Participants With Castration-Resistant Prostate Cancer Previously Treated With Docetaxel Chemotherapy. Identifier: NCT01485861. Retrieved August 4, 2020 from https://clinicaltrials.gov/ct2/show/NCT01485861.
Armstrong AJ, Halabi S, Healy P, Alumkal JJ, Winters C, Kephart J, et al. Phase II trial of the PI3 kinase inhibitor buparlisib (BKM-120) with or without enzalutamide in men with metastatic castration resistant prostate cancer. Eur J Cancer. 2017;81:228–36.
Lin J, Sampath D, Nannini MA, Lee BB, Degtyarev M, Oeh J, et al. Targeting activated Akt with GDC-0068, a novel selective Akt inhibitor that is efficacious in multiple tumor models. Clin Cancer Res. 2013;19:1760–72.
Saura C, Roda D, Rosello S, Oliveira M, Macarulla T, Perez-Fidalgo JA, et al. A first-in-human phase I study of the ATP-competitive AKT inhibitor ipatasertib demonstrates robust and safe targeting of AKT in patients with solid tumors. Cancer Discov. 2017;7:102–13.
De Bono JS, Giorgi UD, Rodrigues DN, Massard C, Bracarda S, Font A, et al. Randomized phase II study evaluating Akt blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with or without PTEN loss. Clin Cancer Res. 2019;25:928–36.
De Bono J, Bracarda S, Sternberg CN, et al. IPATential150: Phase III study of ipatasertib plus abiraterone vs placebo plus abi in metastatic castration resistant prostate cancer. ESMO; 2020.
ClinicalTrials.gov. National Library of Medicine (U.S.). (2000, February 29–). PI3Kbeta Inhibitor AZD8186 and Docetaxel in Treating Patients Advanced Solid Tumors With PTEN or PIK3CB Mutations That Are Metastatic or Cannot Be Removed by Surgery. Identifier: NCT03218826. Retrieved August 4, 2020 from https://clinicaltrials.gov/ct2/show/NCT03218826.
Lin W, Chen Y, Zen L, Ying R, Zhu F. Effect of a novel EZH2 inhibitor GSK126 on prostate cancer cells. J Zhejiang Univ. 2016;45:356–63.
Bai Y, Zhang Z, Cheng L, Wang R, Chen X, Kong Y, et al. Inhibition of enhancer of zeste homolog 2 (EZH2) overcomes enzalutamide resistance in castration-resistant prostate cancer. J Biol Chem. 2019;294:9911–23.
ClinicalTrials.gov. Study of Tazemetostat together with enzalutamide or with abiraterone/prednisone in subjects with castration resistant prostate cancer that has spread who have not yet received chemotherapy. ClinicalTrials.gov identifier (NCT number): NCT04179864.
ClinicalTrials.gov. ProSTAR: a study evaluating CPI-1205 in patients with metastatic castration resistant prostate cancer. ClinicalTrials.gov Identifier: NCT03480646.
Taplin ME, Hussain A, Shah S, Shore N, Edenfield WJ, Sartor OA, et al. Abstract CT094: Phase IB results of ProSTAR: CPI-1205, EZH2 inhibitor, combined with enzalutamide (E) or abiraterone/prednisone (A/P) in patients with metastatic castration resistant prostate cancer (mCRPC). Proceedings of the American Association for Cancer Research Annual Meeting 2019; Atlanta, GA. Philadelphia (PA): AACR; Cancer Res 2019;79(13 Suppl).
Qui X, Wang W, Li B, Cheng B, Lin K, Bai J, et al. Targeting EZH2 could overcome docetaxel resistance in prostate cancer cells. BMC Cancer. 2019;19:27.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Labriola, M.K., Atiq, S., Hirshman, N. et al. Management of men with metastatic castration-resistant prostate cancer following potent androgen receptor inhibition: a review of novel investigational therapies. Prostate Cancer Prostatic Dis 24, 301–309 (2021). https://doi.org/10.1038/s41391-020-00299-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-020-00299-9
This article is cited by
-
Evaluation of the tolerability and safety of [225Ac]Ac-PSMA-I&T in patients with metastatic prostate cancer: a phase I dose escalation study
BMC Cancer (2024)
-
Long-term survival outcomes of salvage [225Ac]Ac-PSMA-617 targeted alpha therapy in patients with PSMA-expressing end-stage metastatic castration-resistant prostate cancer: a real-world study
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
Vulpinic acid as a natural compound inhibits the proliferation of metastatic prostate cancer cells by inducing apoptosis
Molecular Biology Reports (2021)