Abstract
Background
To evaluate the additive role of Ga-68 PSMA PET as a primary staging tool in patients bearing prostate cancer in single PIRADS 4 or 5 index lesions.
Methods
Eighty-one biopsy-naive patients with preoperative mpMRI and Ga-68 PSMA PET who underwent radical prostatectomy (RP) were evaluated retrospectively. Forty-nine patients had PIRADS 4 and 32 had PIRADS 5 index lesions. The localization, grade, and volumetric properties of dominant (DT) and non-dominant tumors (NDT) in RP were compared to the index lesions of mpMRI and Ga-68 PSMA PET.
Results
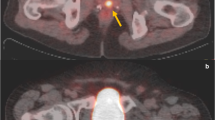
The median age and PSA level were 62 (IQR; 59–69) years and 7 (IQR; 2–8) ng/ml, respectively. Ga-68 PSMA PET detected DTs in 100% of the patients including 13 patients in whom mpMR failed. In 45 patients an NDT was reported in RP. Ga-68 PSMA PET accurately detected NDT in 24 of 45 (53.3%) patients. Six patients (12.2%) in PIRADS 4 and 8 (25%) in PIRADS 5 group showed upgrading. In PIRADS 4, Ga-68 PSMA PET localized DT in all patients with upgraded tumors whereas mpMRI missed exact location in 2 of 6 (33.3%). In PIRADS 5 both mpMRI and Ga-68 PSMA PET accurately located all DTs. Overall detection rates of extracapsular extension (ECE) and seminal vesicle invasion (SVI) by mpMRI were 51.1% and 53.8%, respectively. Ga-68 PSMA PET detected ECE and SVI in 27.9% and 30.7%, respectively. When mpMRI and Ga-68 PSMA PET were used in combination detection rates of ECE and SVI increased to 65.1 and 61.5%. Ga-68 PSMA PET-detected six of ten patients with positive lymph nodes whereas mpMRI could not identify any.
Conclusions
Ga-68 PSMA PET has a better diagnostic accuracy in detecting DT, NDT, upgrading, adverse pathology in patients with PIRADS 4 index lesions. However, mpMRI better predicted ECE and SVI than Ga-68 PSMA PET.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Mottet N, van den Bergh RCN, Briers E, Cornford P, Santis M, Fanti S, et al. EAU-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer 2020. European Association of Urology Guidelines 2020 edition. Presented at the EAU Annual Congress Amsterdam 2020. European Association of Urology Guidelines Office: Arnhem, The Netherlands; 2020.
Esen T, Kilic M, Seymen H, Acar O, Demirkol MO. Can Ga-68 PSMA PET/CT replace conventional imaging modalities for primary lymph node and bone staging of prostate cancer? Eur Urol Focus. 2020;6:218–20.
Berger I, Annabattula C, Lewis J, Shetty DV, Kam J, Maclean F. et al. (68)Ga-PSMA PET/CT vs. mpMRI for locoregional prostate cancer staging: correlation with final histopathology. Prostate Cancer Prostatic Dis. 2018;21:204–11.
Rhee H, Thomas P, Shepherd B, Gustafson S, Vela I, Russell PJ, et al. Prostate specific membrane antigen positron emission tomography may improve the diagnostic accuracy of multiparametric magnetic resonance imaging in localized prostate cancer. J Urol. 2016;196:1261–7.
Demirci E, Kabasakal L, Sahin OE, Akgun E, Gultekin MH, Doganca T, et al. Can SUVmax values of Ga-68-PSMA PET/CT scan predict the clinically significant prostate cancer? Nucl Med Commun. 2019;40:86–91.
Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–16.
de Rooij M, Hamoen EH, Witjes JA, Barentsz JO, Rovers MM. Accuracy of magnetic resonance imaging for local staging of prostate cancer: a diagnostic meta-analysis. Eur Urol. 2016;70:233–45.
Futterer JJ, Briganti A, De Visschere P, Emberton M, Giannarini G, Kirkham A, et al. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol. 2015;68:1045–53.
Giesel FL, Sterzing F, Schlemmer HP, Holland-Letz T, Mier W, Rius M, et al. Intra-individual comparison of (68)Ga-PSMA-11-PET/CT and multi-parametric MR for imaging of primary prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:1400–6.
Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–95.
Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13:226–35.
Kalapara AA, Nzenza T, Pan HY, Ballok Z, Ramdave S, O’Sullivan R, et al. Detection and localisation of primary prostate cancer using (68) Ga-PSMA PET/CT compared with mpMRI and radical prostatectomy specimens. BJU Int. 2020;126:83–90.
Amin A, Blazevski A, Thompson J, Scheltema MJ, Hofman MS, Murphy D, et al. Protocol for the PRIMARY clinical trial, a prospective, multicentre, cross-sectional study of the additive diagnostic value of gallium-68 prostate-specific membrane antigen positron-emission tomography/computed tomography to multiparametric magnetic resonance imaging in the diagnostic setting for men being investigated for prostate cancer. BJU Int. 2020;125:515–24.
Bahler CD, Green M, Hutchins GD, Cheng L, Magers MJ, Fletcher J, et al. Prostate specific membrane antigen targeted positron emission tomography of primary prostate cancer: assessing accuracy with whole mount pathology. J Urol. 2020;203:92–9.
Stabile A, Giganti F, Rosenkrantz AB, Taneja SS, Villeirs G, Gill IS, et al. Multiparametric MRI for prostate cancer diagnosis: current status and future directions. Nat Rev Urol. 2020;17:41–61.
Schieda N, Quon JS, Lim C, El-Khodary M, Shabana W, Singh V, et al. Evaluation of the European Society of Urogenital Radiology (ESUR) PI-RADS scoring system for assessment of extra-prostatic extension in prostatic carcinoma. Eur J Radiol. 2015;84:1843–8.
Roethke MC, Lichy MP, Jurgschat L, Hennenlotter J, Vogel U, Schilling D, et al. Tumorsize dependent detection rate of endorectal MRI of prostate cancer—a histopathologic correlation with whole-mount sections in 70 patients with prostate cancer. Eur J Radiol. 2011;79:189–95.
Zamboglou C, Drendel V, Jilg CA, Rischke HC, Beck TI, Schultze-Seemann W, et al. Comparison of (68)Ga-HBED-CC PSMA-PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics. 2017;7:228–37.
Woythal N, Arsenic R, Kempkensteffen C, Miller K, Janssen JC, Huang K, et al. Immunohistochemical validation of PSMA expression measured by (68)Ga-PSMA PET/CT in primary prostate cancer. J Nucl Med. 2018;59:238–43.
Koerber SA, Utzinger MT, Kratochwil C, Kesch C, Haefner MF, Katayama S. et al. (68)Ga-PSMA-11 PET/CT in newly diagnosed carcinoma of the prostate: correlation of intraprostatic PSMA uptake with several clinical parameters. J Nucl Med. 2017;58:1943–8.
Uprimny C, Kroiss AS, Decristoforo C, Fritz J, von Guggenberg E, Kendler D. et al. (68)Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging. 2017;44:941–9.
Qi F, Zhu K, Cheng Y, Hua L, Cheng G. How to pick out the “Unreal” gleason 3 + 3 patients: a nomogram for more precise active surveillance protocol in low-risk prostate cancer in a Chinese population. J Invest Surg. 2019;6:1–8.
Vellekoop A, Loeb S, Folkvaljon Y, Stattin P. Population based study of predictors of adverse pathology among candidates for active surveillance with Gleason 6 prostate cancer. J Urol. 2014;191:350–7.
Druskin SC, Liu JJ, Young A, Feng Z, Dianat SS, Ludwig WW, et al. Prostate MRI prior to radical prostatectomy: effects on nerve sparing and pathological margin status. Res Rep Urol. 2017;9:55–63.
Rocco B, Sighinolfi MC, Sandri M, Eissa A, Elsherbiny A, Zoeir A, et al. Is extraprostatic extension of cancer predictable? A review of predictive tools and an external validation based on a large and a single center cohort of prostate cancer patients. Urology. 2019;129:8–20.
von Klot CJ, Merseburger AS, Boker A, Schmuck S, Ross TL, Bengel FM. et al. (68)Ga-PSMA PET/CT imaging predicting intraprostatic tumor extent, extracapsular extension and seminal vesicle invasion prior to radical prostatectomy in patients with prostate cancer. Nucl Med Mol Imaging. 2017;51:314–22.
Herlemann A, Wenter V, Kretschmer A, Thierfelder KM, Bartenstein P, Faber C. et al. (68)Ga-PSMA positron emission tomography/computed tomography provides accurate staging of lymph node regions prior to lymph node dissection in patients with prostate cancer. Eur Urol. 2016;70:553–7.
Budaus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial experience of (68)Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. 2016;69:393–6.
Hope TA, Goodman JZ, Allen IE, Calais J, Fendler WP, Carroll PR. Metaanalysis of (68)Ga-PSMA-11 PET accuracy for the detection of prostate cancer validated by histopathology. J Nucl Med. 2019;60:786–93.
van Leeuwen PJ, Emmett L, Ho B, Delprado W, Ting F, Nguyen Q, et al. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int. 2017;119:209–15.
Hovels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63:387–95.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koseoglu, E., Kordan, Y., Kilic, M. et al. Diagnostic ability of Ga-68 PSMA PET to detect dominant and non-dominant tumors, upgrading and adverse pathology in patients with PIRADS 4–5 index lesions undergoing radical prostatectomy. Prostate Cancer Prostatic Dis 24, 202–209 (2021). https://doi.org/10.1038/s41391-020-00270-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-020-00270-8
This article is cited by
-
Comparisons of mpMRI, 68Ga-PSMA PET/CT and mpMRI combined with 68Ga-PSMA PET/CT in diagnosing prostate cancer based on tumor detection, localization and staging
World Journal of Urology (2024)
-
Using PSMA imaging for prognostication in localized and advanced prostate cancer
Nature Reviews Urology (2023)
-
Prebiopsy 68Ga-PSMA PET imaging: can we improve the current diagnostic pathway for prostate cancer?
Prostate Cancer and Prostatic Diseases (2023)
-
PSMA PET/CT and PET/MRI in primary staging of prostate cancer and its effect on patient management
Clinical and Translational Imaging (2023)
-
Head-to-head comparison of prostate-specific membrane antigen PET and multiparametric MRI in the diagnosis of pretreatment patients with prostate cancer: a meta-analysis
European Radiology (2023)