Abstract
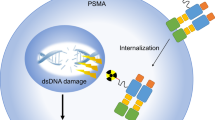
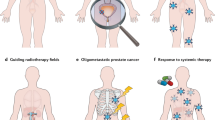
Theranostic principles utilize a molecular biomarker specific for a tumor target, initially for imaging to assess target expression and, if deemed suitable, for targeted therapy. This presents an exciting opportunity for a highly personalized treatment strategy in the era of precision medicine. Prostate-specific membrane antigen (PSMA) theranostics has attracted increasing attention as a promising targeted treatment in metastatic prostate cancer (PC). 177Lu-DOTA-PSMA-617 (177Lu-PSMA-617) is a PSMA-targeted small molecule with favorable properties and is the most extensively investigated PSMA radioligand for radionuclide therapy (RNT) in PC. Since 2014 multiple retrospective studies and more recently a phase II prospective study demonstrated safety and impressive efficacy of 177Lu-PSMA RNT. The evidence generated by these trials led to two currently underway randomized trials in metastatic castrate-resistant PC: TheraP (NCT03392428) and VISION (NCT03511664). While we wait for these pivotal trials to read out, nuclear medicine physicians, medical oncologists, radiation oncologists, and urologists are facing a steep learning curve to master the intricacies and nuances of this novel therapeutic strategy. This review article aims to share and discuss the evolving experience in practical aspects of PSMA theranostics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hertz B. A tribute to Dr. Saul Hertz: the discovery of the medical uses of radioiodine. World J Nucl Med. 2019;18:8–12.
Fahey FH, Grant FD, Thrall JH. Saul Hertz, MD, and the birth of radionuclide therapy. EJNMMI Phys. 2017;4:15.
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of (177)Lu-dotatate for midgut neuroendocrine tumors. New Engl J Med. 2017;376:125–35.
Kwekkeboom DJ, Herder WWd, Kam BL, Eijck CHv, Essen Mv, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30.
Kasperzyk JL, Finn SP, Flavin R, Fiorentino M, Lis R, Hendrickson WK, et al. Prostate-specific membrane antigen protein expression in tumor tissue and risk of lethal prostate cancer. Cancer Epidemiol Biomark Prev. 2013;22:2354–63.
O'Keefe DS, Bacich DJ, Huang SS, Heston WDW. A perspective on the evolving story of PSMA biology, PSMA-based imaging, and endoradiotherapeutic strategies. J Nucl Med. 2018;59:1007–13.
Kratochwil C, Bruchertseifer F, Rathke H, Bronzel M, Apostolidis C, Weichert W, et al. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with (225)Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med. 2017;58:1624–31.
Tagawa ST, Milowsky MI, Morris M, Vallabhajosula S, Christos P, Akhtar NH, et al. Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res. 2013;19:5182–91.
Zechmann CM, Afshar-Oromieh A, Armor T, Stubbs JB, Mier W, Hadaschik B, et al. Radiation dosimetry and first therapy results with a (124)I/ (131)I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41:1280–92.
Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schafers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90.
Heck MM, Tauber R, Schwaiger S, Retz M, D'Alessandria C, Maurer T, et al. Treatment Outcome, Toxicity, and Predictive Factors for Radioligand Therapy with (177)Lu-PSMA-I&T in Metastatic Castration-resistant Prostate Cancer. European urology. 2019;75:920–6.
Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33.
Hofman M, Violet JA, Hicks RJ, Ferdinandus J, Thang SP, Iravani A, et al. Results of a 50 patient single-center phase II prospective trial of Lutetium-177 PSMA-617 theranostics in metastatic castrate-resistant prostate cancer. J Clin Oncol 2019;37:228.
Australian, New Zealand U, Prostate Cancer Trials G, Australian Nuclear S, Technology O, Endocyte, et al. A Trial of 177Lu-PSMA617 Theranostic Versus Cabazitaxel in Progressive Metastatic Castration Resistant Prostate Cancer 2020. https://ClinicalTrials.gov/show/NCT03392428
Endocyte. Study of 177Lu-PSMA-617 In Metastatic Castrate-Resistant Prostate Cancer 2020. https://ClinicalTrials.gov/show/NCT03511664.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–18.
Ahmadzadehfar H, Wegen S, Yordanova A, Fimmers R, Kurpig S, Eppard E, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [(177)Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging 2017;44:1448–54.
Thang SP, Violet J, Sandhu S, Iravani A, Akhurst T, Kong G, et al. Poor Outcomes for Patients with Metastatic Castration-resistant Prostate Cancer with Low Prostate-specific Membrane Antigen (PSMA) Expression Deemed Ineligible for (177)Lu-labelled PSMA Radioligand Therapy. Eur Urol Oncol. 2018
Kwekkeboom DJ, Teunissen JJ, Bakker WH, Kooij PP, de Herder WW, Feelders RA, et al. Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol. 2005;23:2754–62.
Rahbar K, Ahmadzadehfar H, Seifert R, Boegemann M. [(177)Lu]-PSMA-617 radionuclide therapy in patients with metastatic castration-resistant prostate cancer. Lancet Oncol. 2018;19:e371.
Violet J, Jackson P, Ferdinandus J, Sandhu S, Akhurst T, Iravani A, et al. Dosimetry of (177)Lu-PSMA-617 in Metastatic Castration-Resistant Prostate Cancer: Correlations Between Pretherapeutic Imaging and Whole-Body Tumor Dosimetry with Treatment Outcomes. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2019;60:517–23.
Ferdinandus J, Eppard E, Gaertner FC, Kurpig S, Fimmers R, Yordanova A, et al. Predictors of response to radioligand therapy of metastatic castrate-resistant prostate cancer with 177Lu-PSMA-617. J Nucl Med. 2017;58:312–9.
Bailey DL, Hofman MS, Forwood NJ, O'Keefe GJ, Scott AM, van Wyngaardt WM, et al. Accuracy of dose calibrators for (68)Ga PET imaging: unexpected findings in a multicenter clinical pretrial assessment. J Nucl Med. 2018;59:636–8.
Hofman MS, Murphy DG, Williams SG, Nzenza T, Herschtal A, Lourenco RA, et al. A prospective randomized multicentre study of the impact of gallium-68 prostate-specific membrane antigen (PSMA) PET/CT imaging for staging high-risk prostate cancer prior to curative-intent surgery or radiotherapy (proPSMA study): clinical trial protocol. BJU Int. 2018;122:783–93.
Ferreira G, Iravani A, Hofman MS, Hicks RJ. Intra-individual comparison of (68)Ga-PSMA-11 and (18)F-DCFPyL normal-organ biodistribution. Cancer Imaging 2019;19:23.
Giesel FL, Will L, Lawal I, Lengana T, Kratochwil C, Vorster M, et al. Intraindividual Comparison of (18)F-PSMA-1007 and (18)F-DCFPyL PET/CT in the Prospective Evaluation of Patients with Newly Diagnosed Prostate Carcinoma: A Pilot Study. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2018;59:1076–80.
Katsogiannou M, Ziouziou H, Karaki S, Andrieu C, Henry de Villeneuve M, Rocchi P. The hallmarks of castration-resistant prostate cancers. Cancer Treat Rev. 2015;41:588–97.
Lian F, Sharma NV, Moran JD, Moreno CS. The biology of castration-resistant prostate cancer. Curr Probl Cancer. 2015;39:17–28.
Heck MM, Retz M, D'Alessandria C, Rauscher I, Scheidhauer K, Maurer T, et al. Systemic radioligand therapy with (177)Lu labeled prostate specific membrane antigen ligand for imaging and therapy in patients with metastatic castration resistant prostate cancer. J Urol. 2016;196:382–91.
Kratochwil C, Giesel FL, Stefanova M, Benesova M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-Labeled PSMA-617. J Nucl Med. 2016;57:1170–6.
Brauer A, Grubert LS, Roll W, Schrader AJ, Schafers M, Bogemann M, et al. 177)Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1663–70.
Fendler WP, Reinhardt S, Ilhan H, Delker A, Boning G, Gildehaus FJ, et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget. 2017;8:3581–90.
Rahbar K, Boegemann M, Yordanova A, Eveslage M, Schafers M, Essler M, et al. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol Imaging. 2018;45:12–9.
Scarpa L, Buxbaum S, Kendler D, Fink K, Bektic J, Gruber L, et al. The (68)Ga/(177)Lu theragnostic concept in PSMA targeting of castration-resistant prostate cancer: correlation of SUVmax values and absorbed dose estimates. Eur J Nucl Med Mol Imaging. 2017;44:788–800.
Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, Seth A, et al. 177)Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging. 2017;44:81–91.
Emmett L, Crumbaker M, Ho B, Willowson K, Eu P, Ratnayake L, et al. Results of a Prospective Phase 2 Pilot Trial of (177)Lu-PSMA-617 Therapy for Metastatic Castration-Resistant Prostate Cancer Including Imaging Predictors of Treatment Response and Patterns of Progression. Clinical genitourinary cancer. 2019;17:15–22.
Mateo J, Cheng HH, Beltran H, Dolling D, Xu W, Pritchard CC, et al. Clinical outcome of prostate cancer patients with germline DNA repair mutations: retrospective analysis from an international study. Eur Urol. 2018;73:687–93.
Caffo O, Veccia A, Kinspergher S, Rizzo M, Maines F. Aberrations of DNA repair pathways in prostate cancer: future implications for clinical practice? Front Cell Dev Biol. 2018;6:71.
Yordanova A, Linden P, Hauser S, Meisenheimer M, Kurpig S, Feldmann G, et al. Outcome and safety of rechallenge [(177)Lu]Lu-PSMA-617 in patients with metastatic prostate cancer. European journal of nuclear medicine and molecular imaging. 2019;46:1073–80.
Gafita A, Rauscher I, Retz M, Knorr K, Heck M, Wester HJ, et al. Early Experience of Rechallenge (177)Lu-PSMA Radioligand Therapy After an Initial Good Response in Patients with Advanced Prostate Cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2019;60:644–8.
Cheung P. Stereotactic body radiotherapy for oligoprogressive cancer. Br J Radiol. 2016;89:20160251.
Ahmadzadehfar H, Schlolaut S, Fimmers R, Yordanova A, Hirzebruch S, Schlenkhoff C, et al. Predictors of overall survival in metastatic castration-resistant prostate cancer patients receiving [(177)Lu]Lu-PSMA-617 radioligand therapy. Oncotarget. 2017;8:103108–16.
Grubmüller B, Senn D, Kramer G, Baltzer P, D’Andrea D, Grubmüller KH, et al. Response assessment using 68Ga-PSMA ligand PET in patients undergoing 177Lu-PSMA radioligand therapy for metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 2019;46:1063–72.
Taieb D, Foletti JM, Bardies M, Rocchi P, Hicks RJ, Haberkorn U. PSMA-targeted radionuclide therapy and salivary gland toxicity: why does it matter? J Nucl Med. 2018;59:747–8.
Kratochwil C, Giesel FL, Leotta K, Eder M, Hoppe-Tich T, Youssoufian H, et al. PMPA for nephroprotection in PSMA-targeted radionuclide therapy of prostate cancer. J Nucl Med. 2015;56:293–8.
Baum RP, Langbein T, Singh A, Shahinfar M, Schuchardt C, Volk GF, et al. Injection of botulinum toxin for preventing salivary gland toxicity after PSMA radioligand therapy: an empirical proof of a promising concept. Nucl Med Mol Imaging. 2018;52:80–1.
Yilmaz B, Nisli S, Ergul N, Gursu RU, Acikgoz O, Cermik TF. Effect of External Cooling on (177)Lu-PSMA Uptake by the Parotid Glands. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2019;60:1388-93.
Rousseau E, Lau J, Kuo HT, Zhang Z, Merkens H, Hundal-Jabal N, et al. Monosodium glutamate reduces (68)Ga-PSMA-11 uptake in salivary glands and kidneys in a preclinical prostate cancer model. J Nucl Med. 2018;59:1865–8.
British Columbia Cancer A. MSG Use With 18F-DCFPyL PET/CT Imaging 2019 [updated December 31. https://ClinicalTrials.gov/show/NCT03693742
Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5.
Stone P, Richardson A, Ream E, Smith AG, Kerr DJ, Kearney N, et al. Cancer-related fatigue: Inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Ann Oncol. 2000;11:971–5.
Yordanova A, Becker A, Eppard E, Kurpig S, Fisang C, Feldmann G, et al. The impact of repeated cycles of radioligand therapy using [(177)Lu]Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1473–9.
Huang K, Brenner W, Prasad V. Tumor lysis syndrome: a rare but serious complication of radioligand therapies. J Nucl Med. 2019;60:752–5.
Acknowledgements
MSH is supported by a Clinical Fellowship Award from the Peter MacCallum Foundation, with research support from the Movember, Prostate Cancer Foundation of Australia (PCFA), Prostate Cancer Foundtion (PCF) and Department of Defence - Congresionally Directed Medical Research Program (CDRP). For our phase II trial, 177Lu (no carrier added) was supplied by the Australian National Nuclear Science and Technology Organisation (ANSTO) and PSMA-617 was supplied by Advanced Biochemical Compounds (ABX, Radeberg, Germany) and Endocyte, a Novartis company (USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AA reports personal fees from Janssen, grants, personal fees, and nonfinancial support from Astellas, personal fees from Novartis, grants and nonfinancial support from Merck Serono, personal fees from Tolmar, personal fees and nonfinancial support from Amgen, personal fees from Pfizer, personal fees from Bayer, personal fees from Telix Pharmaceuticals, personal fees from Bristol-Myers Squibb and personal fees from Sanofi. MSH reports personal fees and travel reimbursement for delivering educational talks at meetings supported by Ipsen, Sanofi Genzyme, and Jannsen, research support from Endocyte, a Novartis company. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iravani, A., Violet, J., Azad, A. et al. Lutetium-177 prostate-specific membrane antigen (PSMA) theranostics: practical nuances and intricacies. Prostate Cancer Prostatic Dis 23, 38–52 (2020). https://doi.org/10.1038/s41391-019-0174-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-019-0174-x
This article is cited by
-
Radiopharmaceuticals: navigating the frontier of precision medicine and therapeutic innovation
European Journal of Medical Research (2024)
-
Impact of the mouse model and molar amount of injected ligand on the tissue distribution profile of PSMA radioligands
European Journal of Nuclear Medicine and Molecular Imaging (2022)
-
The new international reference system for pure α- and pure β-emitting radionuclides and some electron capture decaying radionuclides by liquid scintillation counting
Journal of Radioanalytical and Nuclear Chemistry (2022)
-
Lutetium Lu 177 Vipivotide Tetraxetan: First Approval
Molecular Diagnosis & Therapy (2022)
-
89Zr-labeled PSMA ligands for pharmacokinetic PET imaging and dosimetry of PSMA-617 and PSMA-I&T: a preclinical evaluation and first in man
European Journal of Nuclear Medicine and Molecular Imaging (2022)