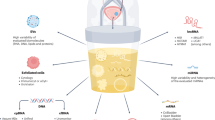
Abstract
Background
Prostate cancer is the most common cancer in American men that ranges from low risk states amenable to active surveillance to high-risk states that can be lethal especially if untreated. There is a critical need to develop relatively non-invasive and clinically useful methods for screening, detection, prognosis, disease monitoring, and prediction of treatment efficacy. In this review, we focus on important advances as well as future efforts needed to drive clinical innovation in this area of urine biomarker research for prostate cancer detection and prognostication.
Methods
We provide a review of current literature on urinary biomarkers for prostate cancer. We evaluate the strengths and limitations of a variety of approaches that vary in sampling strategies and targets measured; discuss reported urine tests for prostate cancer with respect to their technical, analytical, and clinical parameters; and provide our perspectives on critical considerations in approaches to developing a urine-based test for prostate cancer.
Results
There has been an extensive history of exploring urine as a source of biomarkers for prostate cancer that has resulted in a variety of urine tests that are in current clinical use. Importantly, at least three tests have demonstrated high sensitivity (~90%) and negative predictive value (~95%) for clinically significant tumors; however, there has not been widespread adoption of these tests.
Conclusions
Conceptual and methodological advances in the field will help to drive the development of novel urinary tests that in turn may lead to a shift in the clinical paradigm for prostate cancer diagnosis and management.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909–16.
Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. 2008;8:268.
Blute ML Jr, Abel EJ, Downs TM, Kelcz F, Jarrard DF. Addressing the need for repeat prostate biopsy: new technology and approaches. Nat Rev Urol. 2015;12:435.
Julian BA, Suzuki H, Suzuki Y, Tomino Y, Spasovski G, Novak J. Sources of urinary proteins and their analysis by urinary proteomics for the detection of biomarkers of disease. Proteom Clin Appl. 2009;3:1029–43.
Emwas AH, Luchinat C, Turano P, Tenori L, Roy R, Salek RM, et al. Standardizing the experimental conditions for using urine in NMR-based metabolomic studies with a particular focus on diagnostic studies: a review. Metabolomics. 2015;11:872–94.
Prensner JR, Rubin MA, Wei JT, Chinnaiyan AM. Beyond PSA: the next generation of prostate cancer biomarkers. Sci Transl Med. 2012;4:127–123.
Kavanagh JP, Darby C, Costello CB. The response of seven prostatic fluid components to prostatic disease. Int J Androl. 1982;5:487–96.
McCallum KA, Kavanagh JP, Farragher EB, Blacklock NJ. Ratio of post-prostatic massage urinary zinc concentration to initial urinary zinc concentration. Improv Method Assess prostatic Funct Br J Urol. 1988;62:565–70.
Scott WW, Huggins C. The acid phosphatase activity of human urine, an index of prostatic secretion. Endocrinology. 1942;30:107–12.
Breul J, Pickl U, Hartung R. Prostate-specific antigen in urine. Eur Urol. 1994;26:18–21.
Daniel O, Kind PRN, King EJ. Urinary excretion of acid phosphatase. BMJ. 1954;1:19–21.
Moberg PJ, Lizana J, Eneroth P. Analysis of prostatic acid phosphatase in urine voided before and after massage of theprostate in infertile men. Urol Int. 1984;39:189–92.
Grayhack JT, Lee C, Oliver L, Schaeffer AJ, Wendel EF. Biochemical profiles of prostatic fluid from normal and diseased prostate glands. Prostate. 1980;1:227–37.
Costello LC, Franklin RB. Prostatic fluid electrolyte composition for the screening of prostate cancer: a potential solution to a major problem. Prostate Cancer Prostatic Dis. 2009;12:17–24.
Costello LC, Franklin RB. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch Biochem Biophys. 2016;611:100–12.
Medarova Z, Ghosh SK, Vangel M, Drake R, Moore A. Risk stratification of prostate cancer patients based on EPS-urine zinc content. Am J Cancer Res. 2014;4:385–93.
Zhang X-a, Hayes D, Smith SJ, Friedle S, Lippard SJ. New strategy for quantifying biological zinc by a modified zinpyr fluorescence sensor. J Am Chem Soc. 2008;130:15788–9.
Lima AR, Bastos Mde L, Carvalho M, Guedes de Pinho P. Biomarker discovery in human prostate cancer: an update in metabolomics studies. Transl Oncol. 2016;9:357–70.
Kelly RS, Vander Heiden MG, Giovannucci E, Mucci LA. Metabolomic biomarkers of prostate cancer: prediction, diagnosis, progression, prognosis, and recurrence. Cancer Epidemiol, Biomark Prev. 2016;25:887–906.
Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–4.
Jentzmik F, Stephan C, Miller K, Schrader M, Erbersdobler A, Kristiansen G, et al. Sarcosine in urine after digital rectal examination fails as a marker in prostate cancer detection and identification of aggressive tumours. Eur Urol. 2010;58:12–18.
Wu H, Liu T, Ma C, Xue R, Deng C, Zeng H, et al. GC/MS-based metabolomic approach to validate the role of urinary sarcosine and target biomarkers for human prostate cancer by microwave-assisted derivatization. Anal Bioanal Chem. 2011;401:635–46.
Cao DL, Ye DW, Zhu Y, Zhang HL, Wang YX, Yao XD. Efforts to resolve the contradictions in early diagnosis of prostate cancer: a comparison of different algorithms of sarcosine in urine. Prostate Cancer Prostatic Dis. 2011;14:166–72.
Jiang Y, Cheng X, Wang C, Ma Y. Quantitative determination of sarcosine and related compounds in urinary samples by liquid chromatography with tandem mass spectrometry. Anal Chem. 2010;82:9022–7.
Issaq HJ, Veenstra TD. Is sarcosine a biomarker for prostate cancer?. J Sep Sci. 2011;34:3619–21.
Shirasu M, Touhara K. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J Biochem. 2011;150:257–66.
Gordon RT, Schatz CB, Myers LJ, Kosty M, Gonczy C, Kroener J, et al. The use of canines in the detection of human cancers. J Altern Complement Med (New Y, NY). 2008;14:61–67.
Cornu JN, Cancel-Tassin G, Ondet V, Girardet C, Cussenot O. Olfactory detection of prostate cancer by dogs sniffing urine: a step forward in early diagnosis. Eur Urol. 2011;59:197–201.
Taverna G, Tidu L, Grizzi F, Torri V, Mandressi A, Sardella P, et al. Olfactory system of highly trained dogs detects prostate cancer in urine samples. J Urol. 2015;193:1382–7.
Elliker KR, Sommerville BA, Broom DM, Neal DE, Armstrong S, Williams HC. Key considerations for the experimental training and evaluation of cancer odour detection dogs: lessons learnt from a double-blind, controlled trial of prostate cancer detection. BMC Urol. 2014;14:22.
Lippi G, Cervellin G. Canine olfactory detection of cancer versus laboratory testing: myth or opportunity?. Clin Chem Lab Med. 2012;50:435–9.
Jezierski T, Walczak M, Ligor T, Rudnicka J, Buszewski B. Study of the art: canine olfaction used for cancer detection on the basis of breath odour. Perspectives and limitations. J Breath Res. 2015;9:027001.
Hackner K, Pleil J. Canine olfaction as an alternative to analytical instruments for disease diagnosis: understanding ‘dog personality’ to achieve reproducible results. J Breath Res. 2017;11:012001.
Pirrone F, Albertini M. Olfactory detection of cancer by trained sniffer dogs: a systematic review of the literature. J Vet Behav: Clin Appl Res. 2017;19:105–17.
Bax C, Taverna G, Eusebio L, Sironi S, Grizzi F, Guazzoni G, et al. Innovative diagnostic methods for early prostate cancer detection through urine analysis: a review. Cancers. 2018, 10: pii: E123.
Bernabei M, Pennazza G, Santonico M, Corsi C, Roscioni C, Paolesse R, et al. A preliminary study on the possibility to diagnose urinary tract cancers by an electronic nose. Sens Actuators B. 2008;131:1–4.
Roine A, Veskimae E, Tuokko A, Kumpulainen P, Koskimaki J, Keinanen TA, et al. Detection of prostate cancer by an electronic nose: a proof of principle study. J Urol. 2014;192:230–4.
Asimakopoulos AD, Del Fabbro D, Miano R, Santonico M, Capuano R, Pennazza G, et al. Prostate cancer diagnosis through electronic nose in the urine headspace setting: a pilot study. Prostate Cancer Prostatic Dis. 2014;17:206–11.
Krishnan B, Truong LD. Prostatic adenocarcinoma diagnosed by urinary cytology. Am J Clin Pathol. 2000;113:29–34.
Tyler KL, Selvaggi SM. Morphologic features of prostatic adenocarcinoma on ThinPrep(R) urinary cytology. Diagn Cytopathol. 2011;39:101–4.
Varma VA, Fekete PS, Franks MJ, Walther MM. Cytologic features of prostatic adenocarcinoma in urine: a clinicopathologic and immunocytochemical study. Diagn Cytopathol. 1988;4:300–5.
Seybolt JF. Cytology of the urinary tract and prostate. CA Cancer J Clin. 1960;10:129–39.
Sharifi R, Shaw M, Ray V, Rhee H, Nagubadi S, Guinan P. Evaluation of cytologic techniques for diagnosis of prostate cancer. Urology. 1983;21:417–20.
Foot NC, Papanicolaou GN, Holmquist ND, Seybolt JF. Exfoliative cytology of urinary sediments; a review of 2,829 cases. Cancer. 1958;11:127–37.
Nickens KP, Ali A, Scoggin T, Tan SH, Ravindranath L, McLeod DG, et al. Prostate cancer marker panel with single cell sensitivity in urine. Prostate. 2015;75:969–75.
Kobayashi TK, Ueda M, Yamaki T, Yakushiji M. Evaluation of cytocentrifuge apparatus with special reference to the cellular recovery rate. Diagn Cytopathol. 1992;8:420–3.
Echeverry G, Hortin GL, Rai AJ. Introduction to urinalysis: historical perspectives and clinical application. Methods Mol Biol (Clifton, NJ). 2010;641:1–12.
Rupp M, O’Hara B, McCullough L, Saxena S, Olchiewski J. Prostatic carcinoma cells in urine specimens. Cytopathology. 1994;5:164–70.
Fujita K, Pavlovich CP, Netto GJ, Konishi Y, Isaacs WB, Ali S, et al. Specific detection of prostate cancer cells in urine by multiplex immunofluorescence cytology. Hum Pathol. 2009;40:924–33.
Ralla B, Stephan C, Meller S, Dietrich D, Kristiansen G, Jung K. Nucleic acid-based biomarkers in body fluids of patients with urologic malignancies. Crit Rev Clin Lab Sci. 2014;51:200–31.
Tzimagiorgis G, Michailidou EZ, Kritis A, Markopoulos AK, Kouidou S. Recovering circulating extracellular or cell-free RNA from bodily fluids. Cancer Epidemiol. 2011;35:580–9.
Hendriks RJ, van Oort IM, Schalken JA. Blood-based and urinary prostate cancer biomarkers: a review and comparison of novel biomarkers for detection and treatment decisions. Prostate Cancer Prostatic Dis. 2017;20:12–19.
Martignano F, Rossi L, Maugeri A, Galla V, Conteduca V, De Giorgi U, et al. Urinary RNA-based biomarkers for prostate cancer detection. Clin Chim Acta. 2017;473:96–105.
Bussemakers MJ, van Bokhoven A, Verhaegh GW, Smit FP, Karthaus HF, Schalken JA, et al. DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999;59:5975–9.
Sanguedolce F, Cormio A, Brunelli M, D’Amuri A, Carrieri G, Bufo P, et al. Urine TMPRSS2: ERG fusion transcript as a biomarker for prostate cancer: literature review. Clin Genitourin Cancer. 2016;14:117–21.
Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Sci (New Y, NY). 2005;310:644–8.
Laxman B, Tomlins SA, Mehra R, Morris DS, Wang L, Helgeson BE, et al. Noninvasive detection of TMPRSS2:ERG fusion transcripts in the urine of men with prostate cancer. Neoplasia. 2006;8:885–8.
Rogers CG, Yan G, Zha S, Gonzalgo ML, Isaacs WB, Luo J, et al. Prostate cancer detection on urinalysis for alpha methylacyl coenzyme a racemase protein. J Urol. 2004;172:1501–3.
Zehentner BK, Secrist H, Zhang X, Hayes DC, Ostenson R, Goodman G, et al. Detection of alpha-methylacyl-coenzyme-A racemase transcripts in blood and urine samples of prostate cancer patients. Mol Diagn Ther. 2006;10:397–403.
Laxman B, Morris DS, Yu J, Siddiqui J, Cao J, Mehra R, et al. A first-generation multiplex biomarker analysis of urine for the early detection of prostate cancer. Cancer Res. 2008;68:645–9.
Varambally S, Laxman B, Mehra R, Cao Q, Dhanasekaran SM, Tomlins SA, et al. Golgi protein GOLM1 is a tissue and urine biomarker of prostate cancer. Neoplasia. 2008;10:1285–94.
Crocitto LE, Korns D, Kretzner L, Shevchuk T, Blair SL, Wilson TG, et al. Prostate cancer molecular markers GSTP1 and hTERT in expressed prostatic secretions as predictors of biopsy results. Urology. 2004;64:821–5.
Meid FH, Gygi CM, Leisinger HJ, Bosman FT, Benhattar J. The use of telomerase activity for the detection of prostatic cancer cells after prostatic massage. J Urol. 2001;165:1802–5.
Leyten GH, Hessels D, Smit FP, Jannink SA, de Jong H, Melchers WJ, et al. Identification of a candidate gene panel for the early diagnosis of prostate cancer. Clin Cancer Res. 2015;21:3061–70.
Van Neste L, Hendriks RJ, Dijkstra S, Trooskens G, Cornel EB, Jannink SA, et al. Detection of high-grade prostate cancer using a urinary molecular biomarker–based risk score. Eur Urol. 2016;70:740–8.
Rigau M, Ortega I, Mir MC, Ballesteros C, Garcia M, Llaurado M, et al. A three-gene panel on urine increases PSA specificity in the detection of prostate cancer. Prostate. 2011;71:1736–45.
Rigau M, Olivan M, Garcia M, Sequeiros T, Montes M, Colas E, et al. The present and future of prostate cancer urine biomarkers. Int J Mol Sci. 2013;14:12620–49.
Jamaspishvili T, Kral M, Khomeriki I, Vyhnankova V, Mgebrishvili G, Student V, et al. Quadriplex model enhances urine-based detection of prostate cancer. Prostate Cancer Prostatic Dis. 2011;14:354–60.
Ouyang B, Bracken B, Burke B, Chung E, Liang J, Ho SM. A duplex quantitative polymerase chain reaction assay based on quantification of alpha-methylacyl-CoA racemase transcripts and prostate cancer antigen 3 in urine sediments improved diagnostic accuracy for prostate cancer. J Urol. 2009;181:2508–13.
Salami SS, Schmidt F, Laxman B, Regan MM, Rickman DS, Scherr D, et al. Combining urinary detection of TMPRSS2:ERG and PCA3 with serum PSA to predict diagnosis of prostate cancer. Urol Oncol. 2013;31:566–71.
Larsen LK, Jakobsen JS, Abdul-Al A, Guldberg P. Noninvasive detection of high grade prostate cancer by dna methylation analysis of urine cells captured by microfiltration. J Urol. 2018;200:749–57.
Sokoll LJ, Ellis W, Lange P, Noteboom J, Elliott DJ, Deras IL, et al. A multicenter evaluation of the PCA3 molecular urine test: pre-analytical effects, analytical performance, and diagnostic accuracy. Clin Chim Acta. 2008;389:1–6.
Shappell SB, Fulmer J, Arguello D, Wright BS, Oppenheimer JR, Putzi MJ. PCA3 urine mRNA testing for prostate carcinoma: patterns of use by community urologists and assay performance in reference laboratory setting. Urology. 2009;73:363–8.
Wang G, Szeto C-C. Quantification of gene expression in urinary sediment for the study of renal diseases. Nephrology. 2007;12:494–9.
Casadio V, Calistri D, Salvi S, Gunelli R, Carretta E, Amadori D, et al. Urine cell-free DNA integrity as a marker for early prostate cancer diagnosis: a pilot study. Biomed Res Int. 2013;2013:270457.
Rykova EY, Morozkin ES, Ponomaryova AA, Loseva EM, Zaporozhchenko IA, Cherdyntseva NV, et al. Cell-free and cell-bound circulating nucleic acid complexes: mechanisms of generation, concentration and content. Expert Opin Biol Ther. 2012;12(sup1):S141–S153.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83.
Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I, et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2:eCollection 2013.
Principe S, Jones EE, Kim Y, Sinha A, Nyalwidhe JO, Brooks J, et al. In-depth proteomic analyses of exosomes isolated from expressed prostatic secretions in urine. Proteomics. 2013;13:1667–71.
McKiernan J, Donovan MJ, O’Neill V, Bentink S, Noerholm M, Belzer S, et al. A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncol. 2016;2:882–9.
Zhou H, Yuen PS, Pisitkun T, Gonzales PA, Yasuda H, Dear JW, et al. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006;69:1471–6.
Royo F, Zuñiga-Garcia P, Sanchez-Mosquera P, Egia A, Perez A, Loizaga A, et al. Different EV enrichment methods suitable for clinical settings yield different subpopulations of urinary extracellular vesicles from human samples. J Extracell Vesicles 2016, 5:https://doi.org/10.3402/jev.v3405.29497.
Harpole M, Davis J, Espina V. Current state of the art for enhancing urine biomarker discovery. Expert Rev Proteom. 2016;13:609–26.
Adachi J, Kumar C, Zhang Y, Olsen JV, Mann M. The human urinary proteome contains more than 1500 proteins, including a large proportion of membrane proteins. Genome Biol. 2006;7:R80.
Thomas S, Hao L, Ricke WA, Li L. Biomarker discovery in mass spectrometry-based urinary proteomics. Proteom Clin Appl. 2016;10:358–70.
Nagaraj N, Mann M. Quantitative analysis of the intra- and inter-individual variability of the normal urinary proteome. J Proteome Res. 2011;10:637–45.
Pisitkun T, Johnstone R, Knepper MA. Discovery of urinary biomarkers. Mol Cell Proteom. 2006;5:1760–71.
Decramer S, Gonzalez de Peredo A, Breuil B, Mischak H, Monsarrat B, Bascands JL, et al. Urine in clinical proteomics. Mol Cell Proteom. 2008;7:1850–62.
Hendriks Rianne J, Dijkstra S, Jannink Sander A, Steffens Martijn G, van Oort Inge M, Mulders Peter FA, et al. Comparative analysis of prostate cancer specific biomarkers PCA3 and ERG in whole urine, urinary sediments and exosomes. Clin Chem Lab Med. 2016;54:483.
Donovan MJ, Noerholm M, Bentink S, Belzer S, Skog J, O’Neill V, et al. A molecular signature of PCA3 and ERG exosomal RNA from non-DRE urine is predictive of initial prostate biopsy result. Prostate Cancer Prostatic Dis. 2015;18:370–5.
Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, et al. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009;100:1603–7.
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol. 2016;40:244–52.
Truong M, Frye T, Messing E, Miyamoto H. Historical and contemporary perspectives on cribriform morphology in prostate cancer. Nat Rev Urol. 2018;15:475–82.
Deras IL, Aubin SM, Blase A, Day JR, Koo S, Partin AW, et al. PCA3: a molecular urine assay for predicting prostate biopsy outcome. J Urol. 2008;179:1587–92.
Wei JT, Feng Z, Partin AW, Brown E, Thompson I, Sokoll L, et al. Can urinary PCA3 supplement PSA in the early detection of prostate cancer?. J Clin Oncol. 2014;32:4066–72.
Sanda MG, Feng Z, Howard DH, et al. Association between combined TMPRSS2:ERG and PCA3 RNA urinary testing and detection of aggressive prostate cancer. JAMA Oncol. 2017;3:1085–93.
McDunn JE, Li Z, Adam K-P, Neri BP, Wolfert RL, Milburn MV, et al. Metabolomic signatures of aggressive prostate cancer. Prostate. 2013;73:1547–60.
McDunn JE, Stirdivant SM, Ford LA, Wolfert RL. Metabolomics and its application to the development of clinical laboratory tests for prostate cancer. EJIFCC. 2015;26:92–104.
D’Amico A, Santonico M, Pennazza G, Capuano R, Vespasiani G, Del Fabbro D, et al. Approach for prostate cancer diagnosis using a gas sensor array. Procedia Eng. 2012;47:1113–6.
Santonico M, Pennazza G, Asimakopoulos AD, Del Fabbro D, Miano R, Capuano R, et al. Chemical sensors for prostate cancer detection oriented to non-invasive approach. Procedia Eng. 2014;87:320–3.
Zielie PJ, Mobley JA, Ebb RG, Jiang Z, Blute RD, Ho SM. A novel diagnostic test for prostate cancer emerges from the determination of α-methylacyl-coenzyme A racemase in prostatic secretions. J Urol. 2004;172:1130–3.
Rice KR, Chen Y, Ali A, Whitman EJ, Blase A, Ibrahim M, et al. Evaluation of the ETS-related gene mRNA in urine for the detection of prostate cancer. Clin Cancer Res. 2010;16:1572–6.
Talesa VN, Antognelli C, Del Buono C, Stracci F, Serva MR, Cottini E, et al. Diagnostic potential in prostate cancer of a panel of urinary molecular tumor markers. Cancer Biomark: Sect A Dis Markers. 2009;5:241–51.
Wang F, Ren S, Chen R, Lu J, Shi X, Zhu Y, et al. Development and prospective multicenter evaluation of the long noncoding RNA MALAT-1 as a diagnostic urinary biomarker for prostate cancer. Oncotarget. 2014;5:11091–102.
Rigau M, Morote J, Mir MC, Ballesteros C, Ortega I, Sanchez A, et al. PSGR and PCA3 as biomarkers for the detection of prostate cancer in urine. Prostate. 2010;70:1760–7.
Zhu Y, Ren S, Jing T, Cai X, Liu Y, Wang F, et al. Clinical utility of a novel urine-based gene fusion TTTY15-USP9Y in predicting prostate biopsy outcome. Urol Oncol. 2015;33:384.e389–384.e320.
Rogers CG, Gonzalgo ML, Yan G, Bastian PJ, Chan DY, Nelson WG, et al. High concordance of gene methylation in post-digital rectal examination and post-biopsy urine samples for prostate cancer detection. J Urol. 2006;176:2280–4.
Vener T, Derecho C, Baden J, Wang H, Rajpurohit Y, Skelton J, et al. Development of a multiplexed urine assay for prostate cancer diagnosis. Clin Chem. 2008;54:874–82.
Baden J, Green G, Painter J, Curtin K, Markiewicz J, Jones J, et al. Multicenter evaluation of an investigational prostate cancer methylation assay. J Urol. 2009;182:1186–93.
Baden J, Adams S, Astacio T, Jones J, Markiewicz J, Painter J, et al. Predicting prostate biopsy result in men with prostate specific antigen 2.0 to 10.0 ng/ml using an investigational prostate cancer methylation assay. J Urol. 2011;186:2101–6.
Goessl C, Müller M, Heicappell R, Krause H, Straub B, Schrader M, et al. DNA-based detection of prostate cancer in urine after prostatic massage. Urology. 2001;58:335–8.
Cairns P, Esteller M, Herman JG, Schoenberg M, Jeronimo C, Sanchez-Cespedes M, et al. Molecular detection of prostate cancer in urine by GSTP1 hypermethylation. Clin Cancer Res. 2001;7:2727–30.
Jernimo C, Usadel H, Henrique R, Silva C, Oliveira J, Lopes C, et al. Quantitative GSTP1 hypermethylation in bodily fluids of patients with prostate cancer. Urology. 2002;60:1131–5.
Gonzalgo ML, Pavlovich CP, Lee SM, Nelson WG. Prostate cancer detection by GSTP1 methylation analysis of postbiopsy urine specimens. Clin Cancer Res. 2003;9:2673–7.
Minciu R, Dumache R, Gheorghe P, Daminescu L, Rogobete AF, Ionescu D. Molecular diagnostic of prostate cancer from body fluids using methylation-specific PCR (MS-PCR) method. Clin Lab. 2016;62:1183–6.
Woodson K, O’Reilly KJ, Hanson JC, Nelson D, Walk EL, Tangrea JA. The usefulness of the detection of GSTP1 methylation in urine as a biomarker in the diagnosis of prostate cancer. J Urol. 2008;179:508–12.
Payne SR, Serth J, Schostak M, Kamradt J, Strauss A, Thelen P, et al. DNA methylation biomarkers of prostate cancer: confirmation of candidates and evidence urine is the most sensitive body fluid for non-invasive detection. Prostate. 2009;69:1257–69.
Daniunaite K, Jarmalaite S, Kalinauskaite N, Petroska D, Laurinavicius A, Lazutka JR, et al. Prognostic value of RASSF1 promoter methylation in prostate cancer. J Urol. 2014;192:1849–55.
Lombardo ME, Hudson PB. Preliminary evaluation of 5α-reductase type 2 in urine as a potential marker for prostate disease. Steroids. 1997;62:682–5.
Overbye A, Skotland T, Koehler CJ, Thiede B, Seierstad T, Berge V, et al. Identification of prostate cancer biomarkers in urinary exosomes. Oncotarget. 2015;6:30357–76.
Schostak M, Schwall GP, Poznanovic S, Groebe K, Muller M, Messinger D, et al. Annexin A3 in urine: a highly specific noninvasive marker for prostate cancer early detection. J Urol. 2009;181:343–53.
Jedinak A, Curatolo A, Zurakowski D, Dillon S, Bhasin MK, Libermann TA, et al. Novel non-invasive biomarkers that distinguish between benign prostate hyperplasia and prostate cancer. BMC Cancer. 2015;15:259.
Sánchez-Carbayo M, Urrutia M, de Buitrago JMG, Navajo JA. Evaluation of two new urinary tumor markers: bladder tumor fibronectin and cytokeratin 18 for the diagnosis of bladder cancer. Clin Cancer Res. 2000;6:3585–94.
Tanaka M, Tanaka T, Matsuzaki S, Seto Y, Matsuda T, Komori K, et al. Rapid and quantitative detection of human septin family Bradeion as a practical diagnostic method of colorectal and urologic cancers. Med Sci Monit. 2003;9:Mt61–68.
Russo AL, Jedlicka K, Wernick M, McNally D, Kirk M, Sproull M, et al. Urine analysis and protein networking identify met as a marker of metastatic prostate cancer. Clin Cancer Res. 2009;15:4292–8.
Zhang M, Chen L, Yuan Z, Yang Z, Li Y, Shan L, et al. Combined serum and EPS-urine proteomic analysis using iTRAQ technology for discovery of potential prostate cancer biomarkers. Discov Med. 2016;22:281–95.
Lu Q, Zhang J, Allison R, Gay H, Yang W-X, Bhowmick NA, et al. Identification of extracellular δ-catenin accumulation for prostate cancer detection. Prostate. 2008;69:411–8.
Fujita K, Ewing CM, Chan DY, Mangold LA, Partin AW, Isaacs WB, et al. Endoglin (CD105) as a urinary and serum marker of prostate cancer. Int J Cancer. 2009;124:664–9.
Morgan R, Boxall A, Bhatt A, Bailey M, Hindley R, Langley S, et al. Engrailed-2 (EN2): a tumor specific urinary biomarker for the early diagnosis of prostate cancer. Clin Cancer Res. 2011;17:1090–8.
Pandha H, Sorensen KD, Orntoft TF, Langley S, Hoyer S, Borre M, et al. Urinary engrailed-2 (EN2) levels predict tumour volume in men undergoing radical prostatectomy for prostate cancer. BJU Int. 2012;110:E287–E292.
Killick E, Morgan R, Launchbury F, Bancroft E, Page E, Castro E, et al. Role of engrailed-2 (EN2) as a prostate cancer detection biomarker in genetically high risk men. Sci Rep. 2013;3:2059.
Haj-Ahmad TA, Abdalla MAK, Haj-Ahmad Y. Potential urinary protein biomarker candidates for the accurate detection of prostate cancer among benign prostatic hyperplasia patients. J Cancer. 2014;5:103–14.
Kojima Y, Yoneyama T, Hatakeyama S, Mikami J, Sato T, Mori K, et al. Detection of Core2 β-1,6-N-acetylglucosaminyltransferase in post-digital rectal examination urine is a reliable indicator for extracapsular extension of prostate cancer. PLoS ONE. 2015;10:e0138520.
Fujita K, Ewing CM, Isaacs WB, Pavlovich CP. Immunomodulatory IL-18 binding protein is produced by prostate cancer cells and its levels in urine and serum correlate with tumor status. Int J Cancer. 2011;129:424–32.
Stoeber K, Swinn R, Prevost AT, de Clive-Lowe P, Halsall I, Dilworth SM, et al. Diagnosis of genito-urinary tract cancer by detection of minichromosome maintenance 5 protein in urine sediments. J Natl Cancer Inst. 2002;94:1071–9.
Roy R, Louis G, Loughlin KR, Wiederschain D, Kilroy SM, Lamb CC, et al. Tumor-specific urinary matrix metalloproteinase fingerprinting: identification of high molecular weight urinary matrix metalloproteinase species. Clin Cancer Res. 2008;14:6610–7.
Rehman I, Azzouzi AR, Catto JWF, Allen S, Cross SS, Feeley K, et al. Proteomic analysis of voided urine after prostatic massage from patients with prostate cancer: a pilot study. Urology. 2004;64:1238–43.
Müller H, Haug U, Rothenbacher D, Stegmaier C, Brenner H. Evaluation of serum and urinary myeloid related protein-14 as a marker for early detection of prostate cancer. J Urol. 2008;180:1309–13.
Flatley B, Wilmott KG, Malone P, Cramer R. MALDI MS profiling of post-DRE urine samples highlights the potential of β-microseminoprotein as a marker for prostatic diseases. Prostate. 2013;74:103–11.
Bolduc S, Lacombe L, Naud A, Gregoire M, Fradet Y, Tremblay RR. Urinary PSA: a potential useful marker when serum PSA is between 2.5 ng/mL and 10 ng/mL. Can Urol Assoc J. 2007;1:377–81.
Iwakiri J, Grandbois K, Wehner N, Graves HCB, Stamey T. An analysis of urinary prostate specific antigen before and after radical prostatectomy: evidence for secretion of prostate specific antigen by the periurethral glands. J Urol. 1993;149:783–6.
Tremblay J, Frenette G, Tremblay RR, Dupont A, Thabet M, Dube JY. Excretion of three major prostatic secretory proteins in the urine of normal men and patients with benign prostatic hypertrophy or prostate cancer. Prostate. 1987;10:235–43.
Fujita K, Hayashi T, Matsuzaki K, Nakata W, Masuda M, Kawashima A, et al. Decreased fucosylated PSA as a urinary marker for high Gleason score prostate cancer. Oncotarget. 2016;7:56643–9.
Nakayama K, Inoue T, Sekiya S, Terada N, Miyazaki Y, Goto T, et al. The C-Terminal fragment of prostate-specific antigen, a 2331 da peptide, as a new urinary pathognomonic biomarker candidate for diagnosing prostate cancer. PLoS ONE. 2014;9:e107234.
Drake RR, White KY, Fuller TW, Igwe E, Clements MA, Nyalwidhe JO, et al. Clinical collection and protein properties of expressed prostatic secretions as a source for biomarkers of prostatic disease. J Proteom. 2009;72:907–17.
Mitchell PJ, Welton J, Staffurth J, Court J, Mason MD, Tabi Z, et al. Can urinary exosomes act as treatment response markers in prostate cancer?. J Transl Med. 2009;7:4.
Teni TR, Sheth AR, Kamath MR, Sheth NA. Serum and urinary prostatic inhibin-like peptide in benign prostatic hyperplasia and carcinoma of prostate. Cancer Lett. 1988;43:9–14.
Teni TR, Bandivdekar AH, Sheth AR, Sheth NA. Prostatic inhibin-like peptide quantified in urine of prostatic cancer patients by enzyme-linked immunosorbent assay. Clin Chem. 1989;35:1376.
Rosen EM, Joseph A, Jin L, Yao Y, Chau M-HT, Fuchs A, et al. Urinary and tissue levels of scatter factor in transitional cell carcinoma of bladder. J Urol. 1997;157:72–78.
Smith SD, Wheeler MA, Plescia J, Colberg JW, Weiss RM, Altieri DC. Urine detection of survivin and diagnosis of bladder cancer. JAMA. 2001;285:324–8.
Adamson AS, Francis JL, Witherow RON, Snell ME. Urinary tissue factor levels in prostatic carcinoma: a potential. marker of metastatic spread?. Br J Urol. 1993;71:587–92.
Lwaleed BA, Francis JL, Chisholm M. Urinary tissue factor levels in patients with bladder and prostate cancer. Eur J Surg Oncol. 2000;26:44–49.
Fernandez C, Rifai N, Wenger AS, Mickey DD, Silverman LM. A preliminary study of urinary transferrin as a marker for prostatic cancer. Clin Chim Acta. 1986;161:335–9.
van Dieijen-Visser MP, Hendriks MW, Delaere KP, Gijzen AH, Brombacher PJ. The diagnostic value of urinary transferrin compared to serum prostatic specific antigen (PSA) and prostatic acid phosphatase (PAP) in patients with prostatic cancer. Clin Chim Acta. 1988;177:77–80.
Hutchinson LM, Chang EL, Becker CM, Shih M-C, Brice M, DeWolf WC, et al. Use of thymosin β15 as a urinary biomarker in human prostate cancer. Prostate. 2005;64:116–27.
Katafigiotis I, Tyritzis SI, Stravodimos KG, Alamanis C, Pavlakis K, Vlahou A, et al. Zinc alpha2-glycoprotein as a potential novel urine biomarker for the early diagnosis of prostate cancer. BJU Int. 2012;110:E688–693.
Acknowledgements
We acknowledge funding support from the Prostate Cancer Foundation, Urological Research Foundation, and NIH grants U54CA199091, P50CA180995, and X01HG009642 to WJC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
WJC is a consultant/advisory board member for Beckman Coulter and has received commercial research support from Beckman Coulter, DeCode Genetics, and Ohmx. JL, CPP, JNE, and DR declare no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eskra, J.N., Rabizadeh, D., Pavlovich, C.P. et al. Approaches to urinary detection of prostate cancer. Prostate Cancer Prostatic Dis 22, 362–381 (2019). https://doi.org/10.1038/s41391-019-0127-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-019-0127-4
This article is cited by
-
Urinary marker panels for aggressive prostate cancer detection
Scientific Reports (2022)
-
Detection of rare prostate cancer cells in human urine offers prospect of non-invasive diagnosis
Scientific Reports (2022)
-
Nanotechnological strategies for prostate cancer imaging and diagnosis
Science China Chemistry (2022)
-
A novel method for detection of exfoliated prostate cancer cells in urine by RNA in situ hybridization
Prostate Cancer and Prostatic Diseases (2021)
-
Assessment of men’s risk thresholds to proceed with prostate biopsy for the early detection of prostate cancer
International Urology and Nephrology (2019)