Abstract
Background
The contemporary active surveillance (AS) criteria may result in an unsatisfactory misclassification rate, which may delay curative treatment for prostate cancer patients. The magnetic resonance imaging (MRI), not included in any AS criteria, provides useful information for prostate cancer diagnosis. Our goal is to evaluate the diagnostic performance of Prostate Imaging Reporting and Data Systems (PI-RADS) score, a standardized MRI reporting system, in AS candidates enrollment.
Methods
We searched Cochrane CENTRAL, PubMed, and Embase for pertinent studies through June 2018. The standard methods recommended for meta-analyses of diagnostic evaluation were employed. We draw the summary receiver operating characteristic (SROC) curve. Meta-regression analysis was performed to evaluate the effects of confounding factors.
Results
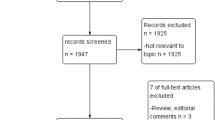
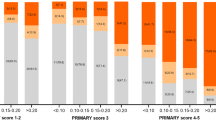
From the resulting 168 studies, 5 provided the diagnostic data on PI-RADS score and pathological results; 834 patients were included. All AS candidates in these studies were defined by Prostate Cancer Research International: Active Surveillance (PRIAS) criterion. The pooled estimates of PI-RADS 4 or 5 on adverse pathological features at radical prostatectomy (RP) among AS candidates were: sensitivity, 0.77 (95% confidence interval (CI), 0.71–0.82); specificity, 0.63 (95% CI, 0.55–0.71); positive predictive value, 0.72 (95% CI, 0.64–0.79); negative predictive value, 0.68 (95% CI, 0.63–0.73); and diagnostic odds ratio, 6 (95% CI, 4–8). The SROC curve was positioned toward the desired upper left corner of the curve, the area under the curve was 0.77 (95% CI, 0.73–0.80). The P-value for heterogeneity was <0.01. The pathological outcomes and endorectal coils contributed to the heterogeneity of sensitivity. The evidences supporting the advantage of PI-RADS v2 over v1 were not sufficient yet.
Conclusion
AS candidates with PI-RADS 4 or 5 may be unsuitable for AS even though they fulfill current AS criteria. Those with PI-RADS 3 or less indicated relative safety for AS enrollment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–31.
Huang GJ, Sadetsky N, Penson DF. Health related quality of life for men treated for localized prostate cancer with long-term followup. J Urol. 2010;183:2206–12.
Dall'Era MA, Albertsen PC, Bangma C, Carroll PR, Carter HB, Cooperberg MR, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012;62:976–83.
Iremashvili V, Pelaez L, Manoharan M, Jorda M, Rosenberg DL, Soloway MS. Pathologic prostate cancer characteristics in patients eligible for active surveillance: a head-to-head comparison of contemporary protocols. Eur Urol. 2012;62:462–8.
Lim SK, Kim KH, Shin TY, Chung BH, Hong SJ, Choi YD, et al. Yonsei criteria: a new protocol for active surveillance in the era of robotic and local ablative surgeries. Clin Genitourin Cancer. 2013;11:501–7.
Kim TH, Jeon HG, Choo SH, Jeong BC, Seo SI, Jeon SS, et al. Pathological upgrading and upstaging of patients eligible for active surveillance according to currently used protocols. Int J Urol. 2014;21:377–81.
Lee SE, Kim DS, Lee WK, Park HZ, Lee CJ, Doo SH, et al. Application of the Epstein criteria for prediction of clinically insignificant prostate cancer in Korean men. BJU Int. 2010;105:1526–30.
Yamada Y, Sakamoto S, Sazuka T, Goto Y, Kawamura K, Imamoto T, et al. Validation of active surveillance criteria for pathologically insignificant prostate cancer in Asian men. Int J Urol. 2016;23:49–54.
Schoots IG, Petrides N, Giganti F, Bokhorst LP, Rannikko A, Klotz L, et al. Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. Eur Urol. 2015;67:627–36.
van den Bergh RC, Ahmed HU, Bangma CH, Cooperberg MR, Villers A, Parker CC. Novel tools to improve patient selection and monitoring on active surveillance for low-risk prostate cancer: a systematic review. Eur Urol. 2014;65:1023–31.
Shukla-Dave A, Hricak H, Akin O, Yu C, Zakian KL, Udo K, et al. Preoperative nomograms incorporating magnetic resonance imaging and spectroscopy for prediction of insignificant prostate cancer. BJU Int. 2012;109:1315–22.
Somford DM, Hambrock T, Hulsbergen-van de Kaa CA, Futterer JJ, van Oort IM, van Basten JP, et al. Initial experience with identifying high-grade prostate cancer using diffusion-weighted MR imaging (DWI) in patients with a Gleason score</=3+3=6 upon schematic TRUS-guided biopsy: a radical prostatectomy correlated series. Invest Radiol. 2012;47:153–8.
Borofsky MS, Rosenkrantz AB, Abraham N, Jain R, Taneja SS. Does suspicion of prostate cancer on integrated T2 and diffusion-weighted MRI predict more adverse pathology on radical prostatectomy? Urology. 2013;81:1279–83.
Lee DH, Koo KC, Lee SH, et al. Tumor lesion diameter on diffusion weighted magnetic resonance imaging could help predict insignificant prostate cancer in patients eligible for active surveillance: preliminary analysis. J Urol. 2013;190:1213–7.
Lee DH, Koo KC, Lee SH, Rha KH, Choi YD, Hong SJ, et al. Low-risk prostate cancer patients without visible tumor (T1c) on multiparametric MRI could qualify for active surveillance candidate even if they did not meet inclusion criteria of active surveillance protocol. Jpn J Clin Oncol. 2013;43:553–8.
Park BH, Jeon HG, Choo SH, Jeong BC, Seo SI, Jeon SS, et al. Role of multiparametric 3.0-Tesla magnetic resonance imaging in patients with prostate cancer eligible for active surveillance. BJU Int. 2014;113:864–70.
Rosenkrantz AB, Prabhu V, Sigmund EE, Babb JS, Deng FM, Taneja SS. Utility of diffusional kurtosis imaging as a marker of adverse pathologic outcomes among prostate cancer active surveillance candidates undergoing radical prostatectomy. AJR Am J Roentgenol. 2013;201:840–6.
Turkbey B, Mani H, Aras O, Ho J, Hoang A, Rastinehad AR, et al. Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology. 2013;268:144–52.
Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69:16–40.
Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–57.
Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36.
McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. Jama. 2018;319:388–96.
Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–35.
Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med. 1993;12:1293–316.
Lijmer JG, Bossuyt PM, Heisterkamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med. 2002;21:1525–37.
Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20:2865–84.
Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–93.
Guo R, Cai L, Fan Y, Jin J, Zhou L, Zhang K. Magnetic resonance imaging on disease reclassification among active surveillance candidates with low-risk prostate cancer: a diagnostic meta-analysis. Prostate Cancer Prostatic Dis. 2015;18:221–8.
Dwamena BA. Evidence-based radiology: step 3--diagnostic systematic review and meta-analysis (critical appraisal). Semin Roentgenol. 2009;44:170–9.
de Cobelli O, Terracciano D, Tagliabue E, Raimondi S, Bottero D, Cioffi A, et al. Predicting pathological features at radical prostatectomy in patients with prostate cancer eligible for active Surveillance by multiparametric magnetic resonance imaging. PloS ONE. 2015;10:e0139696.
Park JJ, Park BK. Role of PI-RADSv2 with multiparametric MRI in determining who needs active surveillance or definitive treatment according to PRIAS. J Magn Reson Imaging. 2017;45:1753–9.
Yim JH, Kim CK. Clinically insignificant prostate cancer suitable for active surveillance according to Prostate Cancer Research International: Active surveillance criteria: Utility of PI-RADS v2. J Magn Reson Imaging. 2018;47:1072–9.
Almeida GL, Petralia G, Ferro M, Ribas CA, Detti S, Jereczek-Fossa BA, et al. Role of multi-parametric magnetic resonance image and PIRADS score in patients with prostate cancer eligible for Active surveillance according PRIAS criteria. Urol Int. 2016;96:459–69.
Porpiglia F, Cantiello F, De Luca S, De Pascale A, Manfredi M, Mele F, et al. Multiparametric magnetic resonance imaging and active surveillance: how to better select insignificant prostate cancer? Int J Urol. 2016;23:752–7.
Porpiglia F, Cantiello F, De Luca S, Manfredi M, Veltri A, Russo F, et al. In-parallel comparative evaluation between multiparametric magnetic resonance imaging, prostate cancer antigen 3 and the prostate health index in predicting pathologically confirmed significant prostate cancer in men eligible for active surveillance. BJU Int. 2016;118:527–34.
Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–7.
Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–13.
Sathianathen NJ, Murphy DG, van den Bergh RC, Lawrentschuk N. Gleason pattern 4: active surveillance no more. BJU Int. 2016;117:856–7.
Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–93.
Becker AS, Cornelius A, Reiner CS, Stocker D, Ulbrich EJ, Barth BK, et al. Direct comparison of PI-RADS version 2 and version 1 regarding interreader agreement and diagnostic accuracy for the detection of clinically significant prostate cancer. Eur J Radiol. 2017;94:58–63.
Turkbey B, Merino MJ, Gallardo EC, Shah V, Aras O, Bernardo M, et al. Comparison of endorectal coil and nonendorectal coil T2W and diffusion-weighted MRI at 3 Tesla for localizing prostate cancer: correlation with whole-mount histopathology. J Magn Reson Imaging. 2014;39:1443–8.
Fan Y, Zhai L, Meng Y, Chen Y, Sun S, Wang H, et al. Contemporary Epstein Criteria with Biopsy-Naïve Multiparametric Magnetic Resonance Imaging to Prevent Incorrect Assignment to Active Surveillance in the PI-RADS Version 2.0 Era. Ann Surg Oncol. 2018;25:3510–7.
Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med. 2018;378:1767–77.
Pal R, Ahmed S, Hannah M, Jaulim A, Walton T, Elkjaer MC, et al. Multi-parametric magnetic resonance imaging monitoring patients in active surveillance for prostate cancer: a prospective cohort study. BJU Int. 2018;52:8–13.
Acknowledgements
This study is supported by the following funds: (1) Youth clinical research project of Peking University First Hospital (Grant No.2017CR07); (2) National Key research and development program of China (Grant No. 2017YFC0908003); (3) Tibetan Natural Science Foundation (Grant No. XZ2017ZR-ZY019).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhai, L., Fan, Y., Meng, Y. et al. The role of Prostate Imaging Reporting and Data System score in Gleason 3 + 3 active surveillance candidates enrollment: a diagnostic meta-analysis. Prostate Cancer Prostatic Dis 22, 235–243 (2019). https://doi.org/10.1038/s41391-018-0111-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-018-0111-4
This article is cited by
-
Machine learning prediction of Gleason grade group upgrade between in-bore biopsy and radical prostatectomy pathology
Scientific Reports (2024)
-
The current role of MRI for guiding active surveillance in prostate cancer
Nature Reviews Urology (2022)
-
Advances in the selection of patients with prostate cancer for active surveillance
Nature Reviews Urology (2021)
-
MRI-derived radiomics model for baseline prediction of prostate cancer progression on active surveillance
Scientific Reports (2021)
-
Prostate Cancer and Prostatic Diseases Best of Asia, 2019: challenges and opportunities
Prostate Cancer and Prostatic Diseases (2020)