Abstract
Background
Up to half of men with Gleason score 6 (GS6) prostate cancers initially managed with active surveillance (AS) will eventually require definitive therapy, usually due to tumor grade reclassification during follow-up. We examined the association between PTEN status on biopsy and subsequent clinicopathologic outcomes in men with GS6 cancers who enrolled in AS.
Methods
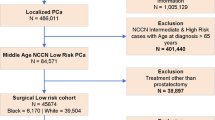
We performed a case–control study of men enrolled in the Johns Hopkins AS cohort with diagnostic biopsy tissue available for immunohistochemical (IHC) staining. IHC was performed for PTEN using genetically validated protocols for all patients. Cases included men who underwent grade reclassification to GS ≥ 3 + 4 = 7 on biopsy within 2 years of follow-up (i.e., early reclassification) or reclassification to GS ≥ 4 + 3 = 7 on biopsy or radical prostatectomy during follow-up (i.e., extreme reclassification). Control patients were diagnosed with GS6 cancer and monitored on AS for at least 8 years without undergoing biopsy reclassification.
Results
Among 67 cases with adequate tissue, 31 men underwent early reclassification and 36 men underwent extreme reclassification. Cases were compared to 65 control patients with adequate tissue for assessment. On initial prostate biopsy, cases were older (median age 67 vs. 65, p = 0.024) and were less likely to meet very-low-risk criteria (64 vs 79%, p = 0.042) as compared to controls. Although not statistically significant, PTEN loss was observed in only 1 (1.5%) of 65 controls as compared to 6 (9%) of 67 cases (p = 0.062).
Conclusions
PTEN loss was rare among men with GS6 prostate cancer enrolled in AS at Johns Hopkins. Despite this, PTEN loss was more frequent among men who underwent early or extreme reclassification to higher-grade cancer as compared to controls. Additional studies in larger low-risk cohorts may better elucidate a potential role for PTEN in selecting patients for AS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Tosoian JJ, Loeb S, Epstein JI, Turkbey B, Choyke PL, Schaeffer EM. Active surveillance of prostate cancer: use, outcomes, imaging, and diagnostic tools. Am Soc Clin Oncol Educ Book. 2016;35:e235–5.
Nassiri N, Margolis DJ, Natarajan S, Sharma DS, Huang J, Dorey FJ, et al. Targeted biopsy to detect Gleason score upgrading during active surveillance for men with low versus intermediate risk prostate cancer. J Urol. 2017;197(3 Pt 1):632–9.
Ma TM, Tosoian JJ, Schaeffer EM, Landis P, Wolf S, Macura KJ, et al. The role of multiparametric magnetic resonance imaging/ultrasound fusion biopsy in active surveillance. Eur Urol. 2017;71:174–80.
Jamaspishvili T, Berman DM, Ross AE, Scher HI, De Marzo AM, Squire JA, et al. Clinical implications of PTEN loss in prostate cancer. Nat Rev Urol. 2018;15:222–34.
Lotan TL, Gurel B, Sutcliffe S, Esopi D, Liu W, Xu J, et al. PTEN protein loss by immunostaining: analytic validation and prognostic indicator for a high risk surgical cohort of prostate cancer patients. Clin Cancer Res. 2011;17:6563–73.
Lotan TL, Carvalho FL, Peskoe SB, Hicks JL, Good J, Fedor HL, et al. PTEN loss is associated with upgrading of prostate cancer from biopsy to radical prostatectomy. Mod Pathol. 2015;28:128–37.
Lotan TL, Wei W, Morais CL, Hawley ST, Fazli L, Hurtado-Coll A, et al. PTEN loss as determined by clinical-grade immunohistochemistry assay is associated with worse recurrence-free survival in prostate cancer. Eur Urol Focus. 2016;2:180–8.
Lotan TL, Heumann A, Rico SD, Hicks J, Lecksell K, Koop C, et al. PTEN loss detection in prostate cancer: comparison of PTEN immunohistochemistry and PTEN FISH in a large retrospective prostatectomy cohort. Oncotarget. 2017;8:65566–76.
Ahearn TU, Pettersson A, Ebot EM, Gerke T, Graff RE, Morais CL. et al. A prospective investigation of PTEN loss and ERG expression in lethal prostate cancer. J Natl Cancer Inst. 2015;108:pii: djv346
Ferraldeschi R, Nava Rodrigues D, Riisnaes R, Miranda S, Figueiredo I, Rescigno P, et al. PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur Urol. 2015;67:795–802.
Reid AH, Attard G, Ambroisine L, Fisher G, Kovacs G, Brewer D, et al. Molecular characterisation of ERG, ETV1 and PTEN gene loci identifies patients at low and high risk of death from prostate cancer. Br J Cancer. 2010;102:678–84.
Mithal P, Allott E, Gerber L, Reid J, Welbourn W, Tikishvili E, et al. PTEN loss in biopsy tissue predicts poor clinical outcomes in prostate cancer. Int J Urol. 2014;21:1209–14.
Lotan TL, Wei W, Ludkovski O, Morais CL, Guedes LB, Jamaspishvili T, et al. Analytic validation of a clinical-grade PTEN immunohistochemistry assay in prostate cancer by comparison with PTEN FISH. Mod Pathol. 2016;29:904–14.
Lokman U, Erickson AM, Vasarainen H, Rannikko AS, Mirtti T. PTEN loss but not ERG expression in diagnostic biopsies is associated with increased risk of progression and adverse surgical findings in men with prostate cancer on active surveillance. Eur Urol Focus 2017.
Tosoian JJ, Mamawala M, Patel HD, Alam R, Epstein JI, Ross AE, et al. Tumor volume on biopsy of low risk prostate cancer managed with active surveillance. J Urol. 2018;199:954–60.
Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL, Committee IG. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;2:1228–42.
Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–7.
Welty CJ, Cowan JE, Nguyen H, Shinohara K, Perez N, Greene KL, et al. Extended followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol. 2015;193:807–11.
Godtman RA, Holmberg E, Khatami A, Pihl CG, Stranne J, Hugosson J. Long-term results of active surveillance in the Göteborg Randomized, Population-based Prostate Cancer Screening Trial. Eur Urol. 2016;70:760–6.
Tosoian JJ, Mamawala M, Epstein JI, Landis P, Wolf S, Trock BJ, et al. Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer. J Clin Oncol. 2015;33:3379–85.
Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990-2013. JAMA. 2015;314:80–82.
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Prostate Cancer (Version 4.2018). https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed September 19, 2018.
Nguyen PL, Haddad Z, Ross AE, Martin NE, Deheshi S, Lam LLC, et al. Ability of a genomic classifier to predict metastasis and prostate cancer-specific mortality after radiation or surgery based on needle biopsy specimens. Eur Urol. 2017;72:845–52.
Nguyen PL, Shin H, Yousefi K, Thompson DJ, Hornberger J, Hyatt AS, et al. Impact of a genomic classifier of metastatic risk on postprostatectomy treatment recommendations by radiation oncologists and urologists. Urology. 2015;86:35–40.
Klein EA, Santiago-Jimenez M, Yousefi K, Robbins BA, Schaeffer EM, Trock BJ, et al. Molecular analysis of low grade prostate cancer using a genomic classifier of metastatic potential. J Urol. 2017;197:122–8.
Tosoian JJ, Chappidi MR, Bishoff JT, Freedland SJ, Reid J, Brawer M, et al. Prognostic utility of biopsy-derived cell cycle progression score in patients with National Comprehensive Cancer Network low-risk prostate cancer undergoing radical prostatectomy: implications for treatment guidance. BJU Int. 2017;120:808–14.
Leapman MS, Cowan JE, Nguyen HG, Shinohara KK, Perez N, Cooperberg MR, et al. Active surveillance in younger men with prostate cancer. J Clin Oncol. 2017;35:1898–904.
Bokhorst LP, Valdagni R, Rannikko A, Kakehi Y, Pickles T, Bangma CH, et al. A decade of active surveillance in the PRIAS Study: an update and evaluation of the criteria used to recommend a switch to active treatment. Eur Urol. 2016;70:954–60.
Tretiakova MS, Wei W, Morais CL, Feng Z, McKenney JK, Simko J, et al. Increased proliferative rate and PTEN loss in prostate cancer are correlated and both associated with risk of recurrence in multivariate models. Mod Pathol. 2016;29:267A.
Guedes LB, Tosoian JJ, Hicks J, Ross AE, Lotan TL. PTEN loss in Gleason Score 3+4=7 prostate biopsies is associated with nonorgan confined disease at radical prostatectomy. J Urol. 2017;197:1054–9.
Baena-Del Valle JA, Zheng Q, Hicks JL, Fedor H, Trock BJ, Morrissey C, et al. Rapid loss of RNA detection by in situ hybridization in stored tissue blocks and preservation by cold storage of unstained slides. Am J Clin Pathol. 2017;148:398–415.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding for this research was provided in part by a Transformative Impact Award from the CDMRP (W81XWH-13-2-0070, to TLL), the Patrick Walsh Prostate Cancer Research Fund, and NCI Cancer Center Support Grant 5P30CA006973.
Conflict of interest
TLL has received research support from Ventana Medical Systems.
Rights and permissions
About this article
Cite this article
Tosoian, J.J., Guedes, L.B., Morais, C.L. et al. PTEN status assessment in the Johns Hopkins active surveillance cohort. Prostate Cancer Prostatic Dis 22, 176–181 (2019). https://doi.org/10.1038/s41391-018-0093-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-018-0093-2
This article is cited by
-
High throughput assessment of biomarkers in tissue microarrays using artificial intelligence: PTEN loss as a proof-of-principle in multi-center prostate cancer cohorts
Modern Pathology (2021)
-
Advances in the selection of patients with prostate cancer for active surveillance
Nature Reviews Urology (2021)
-
Active Surveillance beim Prostatakarzinom
Der Urologe (2019)