Abstract
Background
Although magnetic resonance imaging and subsequent targeted biopsy (‘MRI pathway’) have been widely adopted in routine clinical practice, it is still a common practice to perform systematic biopsy concurrently, because the accuracy of the MRI pathway is yet to be fully defined. This systematic review of the literature assessed the sensitivity of the MRI pathway for detecting clinically significant prostate cancer.
Methods
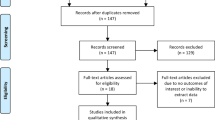
Multiple databases were searched up to May 2017 according to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement for studies assessing the accuracy of MR-guided biopsy (MRGB) compared to a reference standard which consisted of both MRGB and systematic biopsy with at least 20-cores. The primary outcome was the sensitivity of detecting clinically significant prostate cancer defined as Gleason ≥7 disease.
Results
A total of 15 studies met the predefined inclusion criteria. Overall, studies were assessed to be of low quality with inadequate blinding of personnel, which could introduce performance and detection bias. The calculated summary sensitivity of the MRI pathway was 78.3% [95%CI 75.0–81.4%]. There was moderate heterogeneity between the included studies (I2 = 36%). Subgroup analysis was performed based on clinical setting, the strength of MRI magnet and mode of image fusion as factors but no interaction was identified between any of the subgroups. No publication bias was identified.
Conclusion
The MRI pathway cannot yet be solely relied upon to diagnose clinically significant disease and hence additional systematic sampling should still be performed during the biopsy procedure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hodge KK, McNeal JE, Stamey TA. Ultrasound guided transrectal core biopsies of the palpably abnormal prostate. J Urol. 1989;142:66–70.
Turkbey B, Mani H, Shah V, Rastinehad AR, Bernardo M, Pohida T, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186:1818–24.
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–22.
Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390–7.
Thompson J, Stricker P. MRI improves cost and accuracy of prostate cancer biopsy. Nat Rev Urol. 2017;15:6.
Crawford ED, Rove KO, Barqawi AB, Maroni PD, Werahera PN, Baer CA, et al. Clinical–pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate. 2013;73:778–87.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36.
Schwarzer G, Schwarzer MG Package ‘meta’. 2017.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–12.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ Br Med J. 2003;327:557–60.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res. ed.) 1997;315:629–34.
Radtke JP, Kuru TH, Bonekamp D, Freitag MT, Wolf MB, Alt CD, et al. Further reduction of disqualification rates by additional MRI-targeted biopsy with transperineal saturation biopsy compared with standard 12-core systematic biopsies for the selection of prostate cancer patients for active surveillance. Prostate Cancer Prostatic Dis. 2016;19:283–91.
Kuru TH, Roethke MC, Seidenader J, Simpfendorfer T, Boxler S, Alammar K, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190:1380–6.
Radtke JP, Kuru TH, Boxler S, Alt CD, Popeneciu IV, Huettenbrink C, et al. Comparative analysis of transperineal template saturation prostate biopsy versus magnetic resonance imaging targeted biopsy with magnetic resonance imaging-ultrasound fusion guidance. J Urol. 2015;193:87–94.
Hansen NL, Kesch C, Barrett T, Koo B, Radtke JP, Bonekamp D, et al. Multicentre evaluation of targeted and systematic biopsies using magnetic resonance and ultrasound image-fusion guided transperineal prostate biopsy in patients with a previous negative biopsy. BJU Int 2017. 120(5):631–638.
Pepe P, Garufi A, Priolo G, Pennisi M. Transperineal versus transrectal MRI/TRUS fusion targeted biopsy: detection rate of clinically significant prostate cancer. Clin Genitourin Cancer. 2017;15:e33–e6.
Pepe P, Garufi A, Priolo GD, Pennisi M. Multiparametric MRI/TRUS fusion prostate biopsy: advantages of a transperineal approach. Anticancer Res. 2017;37:3291–4.
Distler F, Radtke JP, Kesch C, Roethke M, Schlemmer HP, Roth W, et al. Value of MRI/ultrasound fusion in primary biopsy for the diagnosis of prostate cancer. Urologe. 2016;55:146–55.
Kesch C, Radtke JP, Distler F, Boxler S, Klein T, Huttenbrink C, et al. Multiparametric MRI and MRI-TRUS fusion biopsy in patients with prior negative prostate biopsy. Urol A. 2016;55:1071–7.
Chen K, Tay KJ, Law YM, Aydin H, Ho H, Cheng C, et al. Outcomes of combination MRI-targeted and transperineal template biopsy in restaging low-risk prostate cancer for active surveillance. Asian J Urol. 2017;5:184–193.
Pepe P, Cimino S, Garufi A, Priolo G, Russo GI, Giardina R, et al. Confirmatory biopsy of men under active surveillance: extended versus saturation versus multiparametric magnetic resonance imaging/transrectal ultrasound fusion prostate biopsy. Scandinavian J Urol. 2017;51:260–263.
Filson CP, Natarajan S, Margolis DJ, Huang J, Lieu P, Dorey FJ, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: the role of systematic and targeted biopsies. Cancer. 2016;122:884–92.
Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MM. Magnetic resonance imaging–targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol. 2015;68:438–50.
Evans SM, Nag N, Roder D, Brooks A, Millar JL, Moretti KL, et al. Development of an international prostate cancer outcomes registry. BJU Int. 2016;117(Suppl 4):60–7.
Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-Targeted or standard biopsy for prostate-cancer diagnosis. New Engl J Med. 0:null.
Pokorny MR, de Rooij M, Duncan E, Schroder FH, Parkinson R, Barentsz JO, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol. 2014;66:22–9.
Karakiewicz P, Nazzani S. A valuable tool for prediction of repeat biopsy pathology. Nat Rev Urol. 2017;15:140.
Venderink W, Govers TM, de Rooij M, Futterer JJ, Sedelaar JPM. Cost-effectiveness comparison of imaging-guided prostate biopsy techniques: systematic transrectal ultrasound, direct in-bore MRI, and image fusion. AJR Am J Roentgenol. 2017;208:1058–63.
Shah ZK, Elias SN, Abaza R, Zynger DL, DeRenne LA, Knopp MV, et al. Performance comparison of 1.5-T endorectal coil MRI with 3.0-T nonendorectal coil MRI in patients with prostate cancer. Acad Radiol. 2015;22:467–74.
Riederer SJ, Borisch EA, Froemming AT, Grimm RC, Kawashima A, Mynderse LA, et al. Improved performance of prostate DCE-MRI using a 32-coil vs. 12-coil receiver array. Magn Reson Imaging. 2017;39:15–23.
Vargas HA, Akin O, Franiel T, Mazaheri Y, Zheng J, Moskowitz C, et al. Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology. 2011;259:775–84.
Hamoen EHJ, de Rooij M, Witjes JA, Barentsz JO, Rovers MM. Use of the prostate imaging reporting and data system (PI-RADS) for prostate cancer detection with multiparametric magnetic resonance imaging: a diagnostic meta-analysis. Eur Urol. 2015;67:1112–21.
Gaziev G, Wadhwa K, Barrett T, Koo BC, Gallagher FA, Serrao E, et al. Defining the learning curve for multiparametric magnetic resonance imaging (MRI) of the prostate using MRI-transrectal ultrasonography (TRUS) fusion-guided transperineal prostate biopsies as a validation tool. BJU Int. 2016;117:80–6.
Wegelin O, van Melick HHE, Hooft L, Bosch J, Reitsma HB, Barentsz JO, et al. Comparing three different techniques for magnetic resonance imaging-targeted prostate biopsies: a systematic review of in-bore versus magnetic resonance imaging-transrectal ultrasound fusion versus cognitive registration. Is there a preferred technique? Eur Urol. 2017;71:517–31.
Arsov C, Rabenalt R, Blondin D, Quentin M, Hiester A, Godehardt E, et al. Prospective randomized trial comparing magnetic resonance imaging (MRI)-guided in-bore biopsy to MRI-ultrasound fusion and transrectal ultrasound-guided prostate biopsy in patients with prior negative biopsies. Eur Urol. 2015;68:713–20.
Boesen L, Norgaard N, Logager V, Balslev I, Thomsen HS. Where do transrectal ultrasound- and magnetic resonance imaging-guided biopsies miss significant prostate cancer? Urology. 2017;110:154–60.
Kasivisvanathan V, Dufour R, Moore CM, Ahmed HU, Abd-Alazeez M, Charman SC, et al. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol. 2013;189:860–6.
Lawrence EM, Tang SY, Barrett T, Koo B, Goldman DA, Warren AY, et al. Prostate cancer: performance characteristics of combined T2W and DW-MRI scoring in the setting of template transperineal re-biopsy using MR-TRUS fusion. Eur Radiol. 2014;24:1497–505.
Valerio M, Donaldson I, Emberton M, Ehdaie B, Hadaschik BA, Marks LS, et al. Detection of clinically significant prostate cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: a systematic review. Eur Urol. 2015;68:8–19.
Chen K, Yuen J, Ho H, Cheng C, Lau KOW, Lee LS, et al. Robot-assisted transperineal MRI-ultrasound (MRI-US) fusion targeted biopsy is more efficacious in detecting clinically significant prostate cancer than systematic random saturation biopsy. Int J Urol. 2016;23(Supp 1):64.
Ting F, Van Leeuwen PJ, Thompson J, Shnier R, Moses D, Delprado W, et al. Assessment of the performance of magnetic resonance imaging/ultrasound fusion guided prostate biopsy against a combined targeted plus systematic biopsy approach using 24-core transperineal template saturation mapping prostate biopsy. Prostate cancer. 2016;3794738: 2016.
Acknowledgements
Funding
NJS is supported by a Royal Australasian College of Surgeons (RACS) scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Sathianathen, N.J., Butaney, M., Bongiorno, C. et al. Accuracy of the magnetic resonance imaging pathway in the detection of prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 22, 39–48 (2019). https://doi.org/10.1038/s41391-018-0075-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-018-0075-4
This article is cited by
-
The Evidence for Using Artificial Intelligence to Enhance Prostate Cancer MR Imaging
Current Oncology Reports (2023)
-
Use of multiparametric magnetic resonance imaging (mpMRI) in active surveillance for low-risk prostate cancer: a scoping review on the benefits and harm of mpMRI in different biopsy scenarios
Prostate Cancer and Prostatic Diseases (2021)
-
Genomic and phenotypic heterogeneity in prostate cancer
Nature Reviews Urology (2021)
-
Transrectal versus transperineal prostate biopsy under intravenous anaesthesia: a clinical, microbiological and cost analysis of 2048 cases over 11 years at a tertiary institution
Prostate Cancer and Prostatic Diseases (2021)
-
A systematic review and meta-analysis of Histoscanning™ in prostate cancer diagnostics
World Journal of Urology (2021)