Abstract
Backgroud
The European Organization for Research and Treatment of Cancer (EORTC) trial 22,911 reported 74% 5-year biochemical disease-free survival (bDFS) in patients with prostate carcinoma treated with radical prostatectomy (RP) followed by postoperative radiotherapy (RT). This study aimed to improve these outcomes by using a combined-intensified-modulated-adjuvant treatment, including RT and hormone therapy (HT) after RP.
Materials and methods
This phase I/II trial treatment was designed to improve 5-year bDFS from ~ 75 to 90%. Patients were consecutively enrolled using the following inclusion criteria: age < 80 years, histological diagnosis of prostate adenocarcinoma without known metastases, stage pT2-4N0-1, and Eastern Cooperative Oncology Group performance status of 0–2. All patients had at least one of these pathologic features: capsular perforation, positive surgical margins, seminal vesicle invasion, and pelvic lymph nodes involvement. A minimum dose of 64.8 Gy to the tumor bed was delivered in all patients. Depending on tumor characteristics at diagnosis, patients received a higher dose (70.2 Gy; 85.4%) and/or prophylactic pelvic lymph nodes irradiation (57.7%) and/or HT (69.1%). Biochemical relapse was defined as two consecutive rising prostate-specific antigen (PSA) values > 0.2 ng/ml.
Results
A total of 123 patients were enrolled in the study and completed the scheduled treatment. Median preoperative and postoperative PSA were: 8.8 and 0.06 ng/mL, respectively. The percentages of patients with pathologically involved nodes and positive resection margins were: 14.6% and 58.5%, respectively. With a median follow-up of 67 months (range: 37–120 months), the actuarial 5-year bDFS, local control, metastasis-free survival, and overall survival (OS) were: 92.9%, 98.7%, 96.1%, and 95.1%, respectively.
Conclusion
A higher 5-year bDFS (92.9%) was recorded compared to studies based on standard adjuvant RT, even though patients with nodal disease and detectable postoperative PSA were enrolled. Clinical end points, as long-term disease-free survival and OS, will require further assessments. (ClinicalTrials.gov: NCT03169933)
Similar content being viewed by others
Introduction
Despite a progressive decrease in mortality rates, prostate cancer (PCa) still represents the third cause of cancer-related death in Europe [1]. Radical prostatectomy (RP) is an effective treatment for localized PCa. Nevertheless, a significant percentage of patients (15–60%) develop recurrences after surgery and therefore require salvage radiotherapy (RT) [2,3,4,5,6,7,8]. Several randomized studies have demonstrated the benefit of adjuvant RT after RP in selected patients at high risk of failures [3,4,5].
An improvement in biochemical disease-free survival (bDFS) was first reported by EORTC 22,911 trial in 2005 [9]. The rate of biochemical failure remained significant (25% after 5 years). Based on the results of that study, we hypothesized that RT dose escalation to tumor bed, pelvic lymph node irradiation (PNI) in selected patients with higher risk of regional failures, and adjuvant hormone therapy (HT) for those with a higher risk of distant metastases could further reduce the recurrence rates.
In fact, with a dose higher than 60 Gy on prostatic and seminal vesicles bed, an improved bDFS was previously recorded [10]. In addition, patients at high risk of local failures such as those with positive surgical margins and/or perineural invasion may benefit from further increased doses (up to 70.2 Gy), to minimize recurrence rates [11, 12]. PNI may also reduce regional recurrences in selected patients at high risk for nodal involvement [13]. In fact, some studies have demonstrated an improved bDFS after prophylactic nodal irradiation also in post-prostatectomy setting [14,15,16]. Furthermore, improved bDFS in patients with a high risk of recurrence after RP with the combination of adjuvant HT and RT have been reported [17, 18].
Thus, considering all these factors, we defined combined-intensified-modulated-adjuvant (CIMA) treatment, as a new modality that may potentially improve patients’ outcome, by selectively using RT dose escalation, PNI, and HT based on individual patient risks after RP. The feasibility of CIMA has been previously tested in a preliminary analysis [19]. We now report the long-term outcomes of this study.
Materials and methods
Study objectives
The primary trial objective was to test the possibility to improve 5-year bDFS from 75 to 90%, as calculated from date of surgery to biochemical relapse. Biochemical relapse was defined as two consecutively rising prostate-specific antigen (PSA) values and a PSA level > 0.2 ng/mL. Secondary end points included early and delayed treatment-related side-effects, local control (LC), and metastasis-free survival (MFS). Patients without the events of interest were censored at their last contact date (last PSA assessment).
Study design
A phase I/II trial was planned. A previously published randomized study [9] showed 75% 5-year bDFS in patients treated with standard adjuvant RT (dose: 60 Gy, no PNI, no HT). Considering 90% as the true success rate for our experimental cohort, 100 experimental patients were needed to reject the null hypothesis, that the success rates for CIMA and historical patients are equal with probability (power) 0.8. The 0.05 type I error probability is associated with the test of this null hypothesis. An uncorrected χ2 statistic was used to evaluate this null hypothesis. Some over-recruitment was planned to compensate for 20% drop-out after enrollment.
Inclusion criteria
Patients < 80 years, with resected non-metastatic PCa not previously treated with RT, HT, or chemotherapy (CT) and free from surgical complications were enrolled. Furthermore, patients had at least one of the following risk factors: extracapsular extension, and/or positive surgical margins, and/or seminal vesicle infiltration, and/or regional lymph nodes invasion. Undetectable postoperative PSA was not considered as an inclusion criterion for the study. We used the International Union Against Cancer criteria [20] to define tumor stages. All patients were evaluated by PSA (preoperative and postoperative), abdominal and pelvic CT or MRI, and bone scans prior to enrollment. Patients with distant metastases, extra-pelvic lymphadenopathies, and macroscopic residual disease were excluded. All patients had an Eastern Cooperative Oncology Group (ECOG) performance status between 0 and 2, and adequate bone marrow function (hemoglobin concentration > 8 g/dl, white blood cell count > 3000/mm³, platelet count > 75,000/mm³).
Therapy
Radiotherapy
The details of the three-dimensional (3D) conformal RT technique were described in our previous report [19]. Prior to the planning scans, all patients were given detailed instructions about positioning (supine) and bladder and bowel filling to attain reproducibility during simulation and throughout RT administration. Based on Radiation Therapy Oncology Group (RTOG) guidelines for the definition of the clinical target volume in postoperative conformal RT, we defined two CTVs: CTV1 and CTV2. CTV1 included the prostate and seminal vesicles bed, whereas CTV2 included obturator, internal iliac, external iliac, and presacral (above S2–S3) nodes.
All patients received postoperative RT with set-up evaluation and correction if needed (using Electronic Portal Imaging Device) daily, 5 days a week. We used the International Commission of Radiation Unit 62 guidelines [21] for dose specification and in consideration of tumor characteristics (Table 1), doses were prescribed accordingly: (i) PNI (45 Gy; 1.8 Gy/fraction) plus boost to the prostate bed (19.8–25.2 Gy; 1.8 Gy/fraction; total dose: 64.8–70.2 Gy) or (ii) exclusive prostate bed irradiation (64.8–70.2 Gy; 1.8 Gy/fraction).
Hormone therapy
Table 1 reports HT prescriptions. At commencement of adjuvant RT, patients started either LH-RH analog (leuprorelin, 11.25 mg every 3 months, intramuscularly) or antiandrogen agent (bicalutamide, 150 mg daily per os). Based on risk factors (T stage and Gleason score (GS), to the patients were prescribed short time (6 months) or long time (24 months) HT.
Statistical analysis
A descriptive analysis of the sample was carried out using mean and standard deviation for continuous variables, whereas absolute and relative frequencies for qualitative ones. Patients were monitored weekly during RT. Acute side-effects were scored according to the RTOG scale [22]. Late complications were assessed with the Late Radiation Morbidity Scoring Scheme of the RTOG/European Organization for Research and Treatment of Cancer (EORTC) [22]. Clinical assessment included serum PSA level and digital rectal exam every 3 months for the first 2 years, biannually in 3rd, 4th, and 5th years, and annually thereafter. Additional studies such as bone scans or CT/MRI were requested if there were clinical suspicions of recurrences or increasing PSA levels. Analyzed variables were: age at diagnosis (≤ 65 vs. > 65), pathological evaluation on the extent of the primary tumor (pT2 vs. pT3–4), pathological evaluation of regional lymph nodes (pN0 vs. pN1 vs. pNx), margin status (R0 vs. R1), perineural infiltration (no vs. yes), PSA pre-surgery (≤ 10 ng/mL vs. > 10 ng/mL), PSA post surgery (≤ 0.2 ng/mL vs. > 0.2 ng/mL), histopathologic grade (GS ≤ 7 vs. GS 8–10), lymphadenectomy (no vs. yes), surgical bed dose (64.8 vs. 70.2 Gy), PNI (no vs. yes), HT (no vs. yes), type of HT (antiandrogen vs. LH-RH analog), and duration of HT (short-term: 6 months vs. long time: 24 months). We evaluated the impact of these factors on bDFS. Furthermore, analysis of bDFS, LC, MFS, and overall survival (OS) was performed. Survival curves were calculated with the Kaplan–Meier product-limit method and stratifications for selected prognostic factors were assessed for statistical significance using the log-rank test statistic [23, 24]. Statistical analysis was carried out using SYSTAT, version 11.0 (SPSS, Chicago, IL). A two-sided p value of 0.05 was considered statistically significant.
Ethical issues
All patients consented to treatment and provided a written informed consent to enrollment in the clinical trial. Our institutional review board approved the study. Patients were enrolled from 2004 to 2009. The study is registered in an international public registry (ClinicalTrials.gov Identifier: NCT03169933).
Results
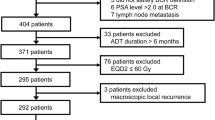
Median follow-up was 67 months (range 37–120 months). Figure 1 illustrates the Consolidated Standards of Reporting Trials (CONSORT) diagram. Patients and treatment characteristics are listed in Table 2. Histologically proven regional lymph nodes invasion (pN1) was 18 (14.6%). Bladder and rectum tumor invasion (pT4) was recorded in four (3.3%) patients. Detectable PSA level (> 0.2 ng/mL) was recorded in nine (7.3%) patients. Five-year LC, MFS, and OS were: 98.7%, 96.1%, and 95.1%, respectively. Actuarial 5-year and 10-year bDFS were 92.9% and 75.8%, respectively (Fig. 2). There was a significant difference between patients with GS ≤ 7 vs. GS > 7 (5-year bDFS: 95.5% vs 78.3 %; p = 0.001) (Table 3, Fig. 3). This difference maintained statistical significance (p = 0.014) even after Bonferroni’s correction for multiple comparisons. Grade 1–2 and Grade 3 acute GI toxicities were recorded in 56 (45.6%) and 3 (2.4%) patients, respectively. Grade 1–2 acute GU toxicities were recorded in 59 (48.0%) patients and Grade 3 GU toxicity in 4 (3.3%) patients, respectively. No patient had Grade 4 acute toxicity. Grade 1 and 2 late GI toxicities were recorded in 15 (12.2%) and 5 (4.1%) patients, respectively. No patient had Grade ≥ 3 GI toxicities. Five-year survival free from Grade 1 and Grade 2 GI toxicities were 87.0% and 96.7%, respectively. Grade 1, Grade 2, and Grade 3 late GU toxicities were recorded in 22 (17.9%), 16 (13.0%), and 5 (4.1%) patients, respectively. Five-year survival free from Grade 1, Grade 2, and Grade 3 GU toxicities were 78.6%, 88.6%, and 95.0%, respectively. No significant differences in terms of Grade ≥ 2 GU and GI toxicities were recorded based on dose to prostate bed, PNI, and adjuvant HT (data not shown).
Discussion
To our knowledge, this is the first prospective study suggesting the possibility to achieve higher bDFS rates by using a tailored treatment after RP for localized PCa. Despite poor prognostic features such as high rates of positive margins and perineural invasion, and inclusion of patients with pathologically involved pelvic nodes, our bDFS seems significantly higher (92.9%) compared with EORTC trial 22,911 [5] and other randomized trials with a biochemical recurrence rate of ~ 25% [3, 4]. Therefore, we could hypothesis that CIMA may improve patient outcomes by a combination of factors as discussed below. Obviously, this conclusion should be considered with caution, as (i) our study was a single arm trial, (ii) the apparent improvement of the results derives from a comparison with different studies. Therefore, we cannot rule out if the “Gleason grade migration” phenomena could have influenced on our comparisons result. In the EORTC 22,911 trial [9], for example, patients were enrolled between 1992 and 2001, clearly earlier compared with our study (2004–2009). Furthermore, comparing our experience with previous studies, we need to consider the RT technological evolution in recent years, which could have also influenced on the results. From the above-mentioned trial of Bolla et al. [9], RT was delivered with 2D technique, whereas in our study, 3D conformal technique was used.
When we analysed the three reported randomized studies [3,4,5] with radiation doses ranging from 60 to 64 Gy, most of our patients (85.4%) received a significantly higher dose (70.2 Gy) to the tumor bed. In addition, patients at risk of pelvic failures underwent PNI, which may be the reason of lower regional recurrences rate at this site, contrary to other studies. We believe that a combination of higher radiation doses with selective PNI may explain the comparable bDFS among patients with R0 vs. R1, and pN1 vs. pN0 disease, respectively. Furthermore, our results suggest the possibility to achieve an improved outcome after PNI compared with prostate irradiation alone as reported in other analyzes [14,15,16] in patients with metastatic pelvic nodes or high pelvic failure risk. It is noteworthy that, despite a higher tumor dose and selective PNI, the rate of acute and long-term toxicity was very low. Table 4 summarizes the results of randomized trials on postoperative RT in comparison with our series. Although, Table 4 clearly shows that the present study achieved a higher 5-year bDFS, we acknowledge the difficulties in comparing these results directly. In fact, our series has the highest rate of patients with lymph node metastases and high Gleason score, but at the same time the lower rate of R1 patients, whereas the proportion of patients with pre-surgical PSA > 10 ng/ml is poorly comparable and that of patients with post-surgical PSA > 0.2 ng/ml is similar to that of randomized trials.
Other non-randomized studies using higher than standard dose ± HT and PNI were published [25,26,27,28]. Table 5 summarizes the results of these series compared with our trial. Although it is difficult to compare those series owing to heterogeneity in terms of margin status and pathological nodal stage, some of these studies seem to confirm that RT dose escalation in high-risk patients after RP may improve bDFS. Cozzarini and colleagues [25] reported 83.0% and 71.0% 5-year bDFS in patients receiving higher and lower than 70.2 Gy RT dose, respectively. This positive impact of dose escalation was also observed in R1 patients [27]. Furthermore, Ost et al. [25], prescribing a dose of 70–77 Gy, reported, 84% 7-year bDFS.
The influence of HT on bDFS after RP is difficult to assess owing to the variations in patients’ selection among retrospective studies [25,26,27,28]. We observed no significant effect of HT on bDFS in our study (5-year bDFS: 91.3% vs 93.7% in patients not receiving or receiving HT, respectively; p 0.486). We could hypothesize that this lack of advantage is due to HT prescription inhomogeneity. Indeed, the use of both androgen deprivation therapy and antiandrogen treatment (high-dose bicalutamide) may represent a limitation of our study. However, when CIMA trial was planned, the standard policy in our center was to inform patients on available evidence and different side-effects of both HTs and to let them choose. Furthermore, we did not observe any differences between the two HTs in terms of bDFS. Therefore, we postulated that the lack of response to HT may have been due to selective prescription and modulation based on risk factors, with HT prescribed only to higher risk subjects.
More generally, the advantages of combining postoperative RT with adjuvant HT after RP has been previously demonstrated. One study reported improved survival in patients with positive pelvic nodes who received both adjuvant HT and postoperative RT compared with patients receiving adjuvant HT alone [29]. Furthermore, a randomized trial showed that adjuvant systemic therapy based on high-dose bicalutamide may improve survival in patients treated with salvage RT after biochemical recurrence [30].
Despite the advantage of combining RT and HT in the adjuvant treatment of high-risk patients, the outcome of patients with high GS remains poorer. In our trial, a GS of 8–10 was correlated with lower bDFS and MFS compared with patients with GS 6–7. Other systemic therapies such as CT could be useful for these patients with higher risk of metastases. For example, in the setting of not-resected high-risk PCa, Fizazi et al. [31] reported a significant improvement of bDFS by combining CT to HT, compared with HT alone. Therefore, prospective trials to investigate the addition of CT to adjuvant treatment of high-risk PCa seem justified.
Unfortunately, being a single arm trial, our study is not able to provide information on the important problem of selecting patients for adjuvant therapies. However, we believe that our study has important clinical implications. Our results suggest that CIMA may improve bDFS by selective use of dose escalation, PNI, and adjuvant HT, with reasonable complications rates. Prospective clinical trials combining postoperative RT, adjuvant HT, and adjuvant CT to further reduce the risk of systemic relapses should be designed. These trials should be planned to enroll patients with high risk of systemic relapses, particularly patients with high GS.
References
Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2012. Ann Oncol. 2012;23:1044–52.
Bottke D, Wiegel T. Prevention of local recurrence using adjuvant radiotherapy after radical prostatectomy. Indications, results, and side effects. Urol A. 2006;45:1251–4.
Thompson IM Jr, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296:2329–35.
Wiegel T, Bottke D, Steiner U, Siegmann A, Golz R, Störkel S, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen. ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–30.
Bolla M, van Poppel H, Tombal B, Vekemans K, Da Pozzo L, de Reijke TM, et al. European Organisation for Research and Treatment of Cancer, Radiation Oncology and GPNIto-Urinary Groups. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380:2018–27.
Daly T, Hickey BE, Lehman M, Francis DP, See AM. Adjuvant radiotherapy following radical prostatectomy for prostate cancer. Cochrane Database Syst Rev. 2011;12:CD007234.
Thompson IM, Valicenti RK, Albertsen P, Davis BJ, Goldenberg SL, Hahn C, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol. 2013;190:441–9.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. part 1: screPNIng, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–13.
Bolla M, van Poppel H, Collette L, van Cangh P, Vekemans K, Da Pozzo L, et al. European Organization for Research and Treatment of Cancer: postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911). Lancet. 2005;366:572–8.
Valicenti RK, Gomella LG, Ismail M, Mulholland SG, Petersen RO, Corn BW. Effect of higher radiation dose on biochemical control after radical prostatectomy for PT3N0 prostate cancer. Int J Radiat Oncol Biol Phys. 1998;42:501–6.
Ploussard G, Agamy MA, Alenda O, Allory Y, Mouracade P, Vordos D, et al. Impact of positive surgical margins on prostate-specific antigen failure after radical prostatectomy in adjuvant treatment-naïve patients. BJU Int. 2011;107:1748–54.
Aumayr K, Breitegger M, Mazal PR, Koller A, Marberger M, Susani M, et al. Quantification of extraprostatic perineural spread and its prognostic value in pT3a pN0 M0 R0 prostate cancer patients. Prostate. 2011;71:1790–5.
Morikawa LK, Roach M 3rd. Pelvic nodal radiotherapy in patients with unfavourable intermediate and high-risk prostate cancer: evidence, rationale, and future directions. Int J Radiat Oncol Biol Phys. 2011;80:6–16.
Spiotto MT, Hancock SL, King CR. Radiotherapy after prostatectomy: improved biochemical relapse-free survival with whole pelvic compared with prostate bed only for high-risk patients. Int J Radiat Oncol Biol Phys. 2007;69:54–61.
Moghanaki D, Koontz BF, Karlin JD, Wan W, Mukhopadhay N, Hagan MP, et al. Elective irradiation of pelvic lymph nodes during postprostatectomy salvage radiotherapy. Cancer. 2013;119:52–60.
Ramey SJ, Agrawal S, Abramowitz MC, Moghanaki D, Pisansky TM, Efstathiou JA, et al. Multi-institutional evaluation of elective nodal irradiation and/or androgen deprivation therapy with postprostatectomy salvage radiotherapy for prostate cancer. Eur Urol. 2018;74:99–106.
Corn BW, Winter K, Pilepich MV. Does androgen suppression enhance the efficacy of postoperative irradiation? A secondary analysis of RTOG 85-31. Radiation Therapy Oncology Group. Urology. 1999;54:495–502.
Miyake H, Sakai I, Harada K, Hara I, Eto H. Long-term results of adjuvant hormonal therapy plus radiotherapy following radical prostatectomy for patients with pT3N0 or pT3N1 prostate cancer. Int J Urol. 2004;11:397–401.
Mantini G, Fersino S, Alitto AR, Frascino V, Massaccesi M, Fionda B, et al. Intensified adjuvant treatment of prostate carcinoma: feasibility analysis of a phase I/II trial. Biomed Res Int. 2014;2014:480725.
Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition; Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803–4.
International Commission on Radiation Units and Measurements. ICRU Report 62. Prescribing, recording, and reporting photon beam therapy (Supplement to ICRU Report 50). Bethesda, MD, USA: ICRU; 1999.
Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;5:1341–6.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Peto R, Peto J. Asymptotically efficient rank invariant procedures. J R Stat Soc. 1972;135:185–207.
Ost P, Cozzarini C, De Meerleer G, Fiorino C, De Potter B, Briganti A, et al. High-dose adjuvant radiotherapy after radical prostatectomy with or without androgen deprivation therapy. Int J Radiat Oncol Biol Phys. 2012;83:960–5.
Bellavita R, Massetti M, Abraha I, Lupattelli M, Mearini L, Falcinelli L, et al. Conformal postoperative radiotherapy in patients with positive resection margins and/or pT3-4 prostate adenocarcinoma. Int J Radiat Oncol Biol Phys. 2012;84:299–304.
Cozzarini C, Montorsi F, Fiorino C, Alongi F, Bolognesi A, Da Pozzo LF, et al. Need for high radiation dose (>or =70 Gy) in early postoperative irradiation after radical prostatectomy: a single-institution analysis of 334 high-risk node-negative patients. Int J Radiat Oncol Biol Phys. 2009;75:966–74.
Katayama S, Habl G, Kessel K, Edler L, Debus J, Herfarth K, et al. Helical intensity modulated radiotherapy of the pelvic lymph nodes with integrated boost to the prostate bed – initial results of the PLATIN 3 Trial. BMC Cancer. 2014;14:14–20.
Briganti A, Karnes RJ, Da Pozzo LF, Cozzarini C, Capitanio U, Gallina A, et al. Combination of adjuvant hormonal and radiation therapy significantly prolongs survival of patients with pT2-4 pN + prostate cancer: results of a matched analysis. Eur Urol. 2011;59:832–40.
Shipley WU, Seiferheld W, Lukka HR, Major PP, Heney NM, Grignon DJ, et al. NRG Oncology RTOG: Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376:417–28.
Fizazi K, Faivre L, Lesaunier F, Delva R, Gravis G, Rolland F, et al. Hormonal therapy plus docetaxel and estramustine versus hormonal therapy alone for high-risk localized prostate cancer (GETUG 12): a phase 3 randomised controlled trial. Lancet Oncol. 2015;16:787–94.
Acknowledgements
We thank Dayleen De Riggs for her help in editing the manuscript.
Author information
Authors and Affiliations
Contributions
Conception and design: ARA, FD, SilC, SaC, MN, GDAP, and AGM; data Collection, GiaS, FD, BF, ARA VV, VF, GabM, and AGM; Analysis and interpretation of data: AF, AA, MB, and SaC; manuscript writing AGM, GioM, MB, NPM, RS, FC, and VV. All authors read and approved the final manuscript and gave consent to publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mantini, G., Siepe, G., Alitto, A.R. et al. Tailored postoperative treatment of prostate cancer: final results of a phase I/II trial. Prostate Cancer Prostatic Dis 21, 564–572 (2018). https://doi.org/10.1038/s41391-018-0064-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-018-0064-7