Abstract
Background
We tested whether tissue-based analysis of p53 and PTEN genomic status in primary tumors is predictive for subsequent sensitivity to abiraterone and enzalutamide in castration-resistant prostate cancer (CRPC).
Methods
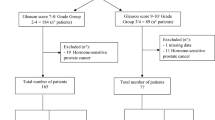
We performed a retrospective analysis of 309 consecutive patients with CRPC treated with abiraterone or enzalutamide. Of these, 101 men (33%) had available primary tumor tissue for analysis. We screened for deleterious TP53 missense mutations and PTEN deletions using genetically validated immunohistochemical assays for nuclear accumulation of p53 protein and PTEN protein loss, with sequencing confirmation of TP53 mutations in a subset. Overall survival (OS) and progression-free survival (PFS) were compared between patients with and without p53 and/or PTEN alterations.
Results
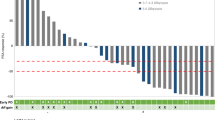
Forty-eight percent of the evaluable cases had PTEN loss and 27% had p53 nuclear accumulation. OS and PFS did not differ according to PTEN status, but were significantly associated with p53 status. Median OS was 16.7 months (95% CI, 14–21.9 months) and 31.2 months (95% CI, 24.5–43.4) for men with and without p53 nuclear accumulation, respectively (HR 2.32; 95% CI 1.19–4.51; P = 0.0018). Similarly, median PFS was 5.5 months (95% CI, 3.2–9.9 months) and 10.9 months (95% CI, 8–15.2 months) in men with and without p53 nuclear accumulation, respectively (HR 2.14, 95%CI 1.20–3.81; P = 0.0008). In multivariable analyses, p53 status was independently associated with PFS (HR 2.15; 95% CI 1.03–4.49; P = 0.04) and a HR of 2.19 for OS (95% CI 0.89–5.40; P = 0.087).
Conclusions
p53 inactivation in the primary tumor (but not PTEN loss) may be predictive of inferior outcomes to novel hormonal therapies in CRPC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Buttigliero C, Tucci M, Bertaglia V, Vignani F, Bironzo P, Di Maio M, et al. Understanding and overcoming the mechanisms of primary and acquired resistance to abiraterone and enzalutamide in castration resistant prostate cancer. Cancer Treat Rev. 2015;41:884–92.
Maughan BL, Antonarakis ES. Androgen pathway resistance in prostate cancer and therapeutic implications. Expert Opin Pharmacother. 2015;16:1521–37.
Jiao J, Wang S, Qiao R, Vivanco I, Watson PA, Sawyers CL, et al. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res. 2007;67:6083–91.
Mulholland DJ, Tran LM, Li Y, Cai H, Morim A, Wang S, et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. 2011;19:792–804.
Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–86.
Ferraldeschi R, Nava Rodrigues D, Riisnaes R, Miranda S, Figueiredo I, Rescigno P, et al. PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur Urol. 2015;67:795–802.
Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell . 2015;161:1215–28.
Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355:84–8.
Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. 2017;355:78–83.
Tan HL, Sood A, Rahimi HA, Wang W, Gupta N, Hicks J, et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin Cancer Res. 2014;20:890–903.
George J, Lim JS, Jang SJ, Cun Y, Ozretic L, Kong G, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524:47–53.
Lee JK, Lee J, Kim S, Kim S, Youk J, Park S, et al. Clonal history and genetic predictors of transformation into small-cell Carcinomas from lung adenocarcinomas. J Clin Oncol. 2017;35:3065–74.
Epstein JI, Amin MB, Beltran H, Lotan TL, Mosquera JM, Reuter VE, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol. 2014;38:756–67.
Aparicio AM, Shen L, Tapia EL, Lu JF, Chen HC, Zhang J, et al. Combined tumor suppressor defects characterize clinically defined aggressive variant prostate cancers. Clin Cancer Res. 2016;22:1520–30.
Aggarwal RR, Youngren J, Sokolov A, Huang J, Thomas GV, True LD, et al. Persistence of AR signaling in small cell neuroendocrine prostate cancer (SCNC) and intermediate atypical carcinoma (IAC): results from the SU2C/PCF/AACR West Coast Prostate Cancer Dream Team (WCDT). J Clin Oncol. 2016;34:5045.
Maughan BL, Luber B, Nadal R, Antonarakis ES. Comparing sequencing of abiraterone and enzalutamide in men with metastatic castration-resistant prostate cancer: a retrospective study. Prostate. 2017;77:33–40.
Maughan BL, Xhou XC, Suzman DL, Nadal R, Bassi S, Schweizer MT, et al. Optimal sequencing of docetaxel and abiraterone in men with metastatic castration-resistant prostate cancer. Prostate. 2015;75:1814–20.
Lotan TL, Wei W, Ludkovski O, Morais CL, Guedes LB, Jamaspishvili T, et al. Analytic validation of a clinical-grade PTEN immunohistochemistry assay in prostate cancer by comparison with PTEN FISH. Mod Pathol. 2016;29:904–14.
Hinds P, Finlay C, Levine AJ. Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J Virol. 1989;63:739–46.
Visakorpi T, Kallioniemi OP, Heikkinen A, Koivula T, Isola J. Small subgroup of aggressive, highly proliferative prostatic carcinomas defined by p53 accumulation. J Natl Cancer Inst. 1992;84:883–7.
Hall MC, Navone NM, Troncoso P, Pollack A, Zagars GK, von Eschenbach AC, et al. Frequency and characterization of p53 mutations in clinically localized prostate cancer. Urology. 1995;45:470–5.
Bauer JJ, Sesterhenn IA, Mostofi KF, McLeod DG, Srivastava S, Moul JW. p53 nuclear protein expression is an independent prognostic marker in clinically localized prostate cancer patients undergoing radical prostatectomy. Clin Cancer Res. 1995;1:1295–300.
Yemelyanova A, Vang R, Kshirsagar M, Lu D, Marks MA, Shih Ie M, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011;24:1248–53.
Kuhn E, Kurman RJ, Vang R, Sehdev AS, Han G, Soslow R, et al. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma--evidence supporting the clonal relationship of the two lesions. J Pathol. 2012;226:421–6.
Guedes LB, Almutairi F, Haffner MC, Rajoria G, Liu Z, Klimek S, et al. Analytic, preanalytic, and clinical validation of p53 IHC for detection of TP53 missense mutation in prostate cancer. Clin Cancer Res. 2017;23:4693-703.
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59.
Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38.
Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Zhu Y, et al. Clinical significance of androgen receptor splice variant-7 mRNA Detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35:2149–56.
Azad AA, Volik SV, Wyatt AW, Haegert A, Le Bihan S, Bell RH, et al. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin Cancer Res. 2015;21:2315–24.
Chen EJ, Sowalsky AG, Gao S, Cai C, Voznesensky O, Schaefer R, et al. Abiraterone treatment in castration-resistant prostate cancer selects for progesterone responsive mutant androgen receptors. Clin Cancer Res. 2015;21:1273–80.
Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer Discov. 2013;3:1030–43.
Balbas MD, Evans MJ, Hosfield DJ, Wongvipat J, Arora VK, Watson PA, et al. Overcoming mutation-based resistance to antiandrogens with rational drug design. Elife . 2013;2:e00499.
Joseph JD, Lu N, Qian J, Sensintaffar J, Shao G, Brigham D, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3:1020–9.
Wyatt AW, Annala M, Parimi S, Zulfiqar M, Finch DL, Oja CD, et al. Circulating tumor DNA (ctDNA) burden and actionable mutations in treatment-naive metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2016;34:5034.
Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell . 2015;163:1011–25.
Kumar A, Coleman I, Morrissey C, Zhang X, True LD, Gulati R, et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat Med. 2016;22:369–78.
Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JM, Papaemmanuil E, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520:353–7.
Haffner MC, Mosbruger T, Esopi DM, Fedor H, Heaphy CM, Walker DA, et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest. 2013;123:4918–22.
Hong MK, Macintyre G, Wedge DC, Van Loo P, Patel K, Lunke S, et al. Tracking the origins and drivers of subclonal metastatic expansion in prostate cancer. Nat Commun. 2015;6:6605.
Lotan TL, Gurel B, Sutcliffe S, Esopi D, Liu W, Xu J, et al. PTEN protein loss by immunostaining: analytic validation and prognostic indicator for a high risk surgical cohort of prostate cancer patients. Clin Cancer Res. 2011;17:6563–73.
Acknowledgments
We are grateful to our patients for their participation in this and other studies and allowing us to be involved in their care.
Author contributions
BLM: Designed the project, performed research, interpreted data, wrote the initial draft, reviewed/approved the final manuscript. LBG: performed research, reviewed/approved the final manuscript. KB: Performed the statistical analysis, reviewed/approved the final manuscript. GR: Provided the reagents, reviewed/approved the final manuscript. ZL: Provided reagents, reviewed/approved the final manuscript. SK: Provided reagents, reviewed/approved the final manuscript. RZ: Provided reagents, reviewed/approved the final manuscript. ESA: Designed the project, interpreted data, reviewed/approved the final manuscript, provided project supervision. TLL: Designed the project, interpreted data, performed research, reviewed/approved the final manuscript, provided project supervision. All authors had access to all of the data and were involved in the writing of the manuscript.
Funding
This work was funded in part by a CDMRP Prostate Cancer Research Program Transformative Impact Award to TLL (W81XWH-12-PCRP-TIA). BLM received funding from the ASCO/Conquer Cancer Foundation (Young Investigator Award).
Statement of relevance
We tested the hypothesis that loss of p53 and/or PTEN in primary tumor specimens is associated with reduced response to subsequent treatment with abiraterone or enzalutamide in patients with CRPC. We find that p53 inactivation, but not PTEN loss, is associated with significantly worse overall and progression-free survival with abiraterone or enzalutamide treatment, suggesting that p53 status is predictive of response to androgen receptor-targeted therapies years later when CRPC develops.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
ESA is a paid consultant/advisor to Janssen, Astellas, Sanofi, Dendreon, Medivation, and Essa. He has received research funding from Janssen, Jonhson & Johnson, Sanofi, Dendreon, Aragon, Exelixis, Genentech, Novartis, and Tokai. GR, ZL, SK, and RZ are employees of Pathline Emerge Pathology Services. The remaining authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Maughan, B.L., Guedes, L.B., Boucher, K. et al. p53 status in the primary tumor predicts efficacy of subsequent abiraterone and enzalutamide in castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 21, 260–268 (2018). https://doi.org/10.1038/s41391-017-0027-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-017-0027-4
This article is cited by
-
Heterogeneity of [68Ga]Ga-PSMA-11 PET/CT in metastatic castration-resistant prostate cancer: genomic characteristics and association with abiraterone response
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
Differential impact of tumor suppressor gene (TP53, PTEN, RB1) alterations and treatment outcomes in metastatic, hormone-sensitive prostate cancer
Prostate Cancer and Prostatic Diseases (2022)
-
Editor’ summary: A paradigm shift in castration-resistant prostate cancer management
Prostate Cancer and Prostatic Diseases (2022)
-
Regulating tumor suppressor genes: post-translational modifications
Signal Transduction and Targeted Therapy (2020)
-
Updates in advanced prostate cancer 2018
Prostate Cancer and Prostatic Diseases (2018)