Abstract
Background
Despite a growing understanding of bronchopulmonary dysplasia (BPD) and advances in management, BPD rates remain stable. There is mounting evidence that BPD may be due to a systemic insult, such as acute kidney injury (AKI). Our hypothesis was that severe AKI would be associated with BPD.
Methods
We conducted a secondary analysis of premature infants [24–27 weeks gestation] in the Recombinant Erythropoietin for Protection of Infant Renal Disease cohort (N = 885). We evaluated the composite outcome of Grade 2/3 BPD or death using generalized estimating equations. In an exploratory analysis, urinary biomarkers of angiogenesis (ANG1, ANG2, EPO, PIGF, TIE2, FGF, and VEGFA/D) were analyzed.
Results
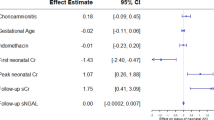
594 (67.1%) of infants had the primary composite outcome of Grade 2/3 BPD or death. Infants with AKI (aOR: 1.69, 95% CI: 1.16–2.46) and severe AKI (aOR: 2.05, 95% CI: 1.19–3.54). had increased risk of the composite outcome after multivariable adjustment Among 106 infants with urinary biomarkers assessed, three biomarkers (VEGFA, VEGFD, and TIE2) had AUC > 0.60 to predict BPD.
Conclusions
Infants with AKI had a higher likelihood of developing BPD/death, with the strongest relationship seen in those with more severe AKI. Three urinary biomarkers of angiogenesis may have potential to predict BPD development.
Impact
-
AKI is associated with lung disease in extremely premature infants, and urinary biomarkers may predict this relationship.
-
Infants with AKI and severe AKI have higher odds of BPD or death. Three urinary angiogenesis biomarkers are altered in infants that develop BPD.
-
These findings have the potential to drive future work to better understand the mechanistic pathways of BPD, setting the framework for future interventions to decrease BPD rates.
-
A better understanding of the mechanisms of BPD development and the role of AKI would have clinical care, cost, and quality of life implications given the long-term effects of BPD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
De‐identified individual participant data are available through the NINDS Data Archive: https://www.ninds.nih.gov/Current‐Research/Research‐ Funded‐NINDS/Clinical‐Research/Archived‐Clinical‐ Research‐Datasets. The data will be de‐identified and a limited access data is available through a request form on that page. Data dictionaries, in addition to study protocol, the statistical analysis plan, and the informed consent form are included. The data will be made available to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal.
References
Baraldi, E., Carraro, S. & Filippone, M. Bronchopulmonary dysplasia: definitions and long-term respiratory outcome. Early Hum. Dev. 85, S1–S3 (2009).
Jensen, E. A. et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. an evidence-based approach. Am. J. Respir. Crit. Care Med. 200, 751–759 (2019).
Bancalari, E. H. & Jobe, A. H. The respiratory course of extremely preterm infants: a dilemma for diagnosis and terminology. J. Pediatr. 161, 585–588 (2012).
Thébaud, B. & Abman, S. H. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am. J. Respir. Crit. Care Med. 175, 978–985 (2007).
Askenazi, D. J. et al. Prevalence of acute kidney injury (Aki) in extremely low gestational age neonates (Elgan). Pediatr. Nephrol. 35, 1737–1748 (2020).
Basu, R. K. & Wheeler, D. S. Kidney-lung cross-talk and acute kidney injury. Pediatr. Nephrol. 28, 2239–2248 (2013).
Starr, M. C. et al. Acute kidney injury and bronchopulmonary dysplasia in premature neonates born less than 32 weeks’ gestation. Am. J. Perinatol. 37, 341–348 (2020).
Askenazi, D. et al. Acute kidney injury is associated with bronchopulmonary dysplasia/mortality in premature infants. Pediatr. Nephrol. 30, 1511–1518 (2015).
Levesque, B. M. et al. Low urine vascular endothelial growth factor levels are associated with mechanical ventilation, bronchopulmonary dysplasia and retinopathy of prematurity. Neonatology 104, 56–64 (2013).
Kang, D. H., Hughes, J., Mazzali, M., Schreiner, G. F. & Johnson, R. J. Impaired angiogenesis in the remnant kidney model: Ii. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J. Am. Soc. Nephrol. 12, 1448–1457 (2001).
Mansour, S. G. et al. The association of angiogenesis markers with acute kidney injury and mortality after cardiac surgery. Am. J. Kidney Dis. 74, 36–46 (2019).
Bose, C. et al. Blood protein concentrations in the first two postnatal weeks that predict bronchopulmonary dysplasia among infants born before the 28th week of gestation. Pediatr. Res. 69, 347–353 (2011).
Augustin, H. G., Koh, G. Y., Thurston, G. & Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin-tie system. Nat. Rev. Mol. Cell Biol. 10, 165–177 (2009).
Ahlfeld, S. K., Gao, Y., Conway, S. J. & Tepper, R. S. Relationship of structural to functional impairment during alveolar-capillary membrane development. Am. J. Pathol. 185, 913–919 (2015).
Juul, S. E. et al. A randomized trial of erythropoietin for neuroprotection in preterm infants. N. Engl. J. Med. 382, 233–243 (2020).
Zappitelli, M. et al. Developing a neonatal acute kidney injury research definition: a report from the niddk neonatal aki workshop. Pediatr. Res. 82, 569–573 (2017).
Starr, M. C. et al. Impact of processing methods on urinary biomarkers analysis in neonates. Pediatr. Nephrol. 33, 181–186 (2018).
Askenazi, D. J. et al. Baseline values of candidate urine acute kidney injury biomarkers vary by gestational age in premature infants. Pediatr. Res. 70, 302–306 (2011).
Lassus, P. et al. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am. J. Respir. Crit. Care Med. 164, 1981–1987 (2001).
Askenazi, D. J. et al. Acute kidney injury urine biomarkers in very low-birth-weight infants. Clin. J. Am. Soc. Nephrol. 11, 1527–1535 (2016).
Liu, K. D. et al. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit. Care 13, R104 (2009).
Gist, K. M. et al. Assessment of the independent and synergistic effects of fluid overload and acute kidney injury on outcomes of critically Ill children. Pediatr. Crit. Care Med. 21, 170–177 (2020).
Baker, C. D. & Abman, S. H. Impaired pulmonary vascular development in bronchopulmonary dysplasia. Neonatology 107, 344–351 (2015).
Surate Solaligue, D. E., Rodríguez-Castillo, J. A., Ahlbrecht, K. & Morty, R. E. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell Mol. Physiol. 313, L1101–l1153 (2017).
Elgendy, M. M. et al. Trends and racial disparities for acute kidney injury in premature infants: the Us national database. Pediatr. Nephrol. 36, 2789–2795 (2021).
Bignall, O. N. R., Harer, M. W., Sanderson, K. R. & Starr, M. C. Commentary on “trends and racial disparities for acute kidney injury in premature infants: the US national database”. Pediatr. Nephrol. 36, 2587–2591 (2021).
D’Angio, C. T. & Maniscalco, W. M. The role of vascular growth factors in hyperoxia-induced injury to the developing lung. Front Biosci. 7, d1609–d1623 (2002).
Meller, S. & Bhandari, V. Vegf levels in humans and animal models with Rds and Bpd: temporal relationships. Exp. Lung Res. 38, 192–203 (2012).
Been, J. V. et al. Early alterations of growth factor patterns in bronchoalveolar lavage fluid from preterm infants developing bronchopulmonary dysplasia. Pediatr. Res. 67, 83–89 (2010).
Balena-Borneman, J. et al. Biomarkers associated with bronchopulmonary dysplasia/mortality in premature infants. Pediatr. Res. 81, 519–525 (2017).
Hu, R., Cheng, Y., Jing, H. & Wu, H. Erythropoietin promotes the protective properties of transplanted endothelial progenitor cells against acute lung injury via Pi3k/Akt pathway. Shock 42, 327–336 (2014).
Vrijlandt, E. J., Gerritsen, J., Boezen, H. M., Grevink, R. G. & Duiverman, E. J. Lung function and exercise capacity in young adults born prematurely. Am. J. Respir. Crit. Care Med. 173, 890–896 (2006).
Acknowledgements
The authors would like to thank Samantha Wallace (Department of Pediatrics, Indiana University School of Medicine) for help with technical editing and proofreading of this manuscript. We would like to thank Lynn Dill, RN, and Emily Pao for their assistance in coordinating the REPaIReD study. We would like to thank the investigators, clinicians, research personnel, study teams, and families who participated in the PENUT Trial. This study was supported by grants R01DK103608, U01NS077955, and U01NS077953. Funding sources for this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Consortia
Contributions
Dr. Starr conceptualized and designed this study, assisted with biomarker performance and data analysis, drafted the initial manuscript, and reviewed and revised the manuscript. Mr. Schmicker and Halloran performed biomarker and data analysis and critically revised the manuscript. Drs. Heagerty, Brophy, Goldstein, and Juul made substantial contributions to the conception of this project and reviewed and revised the manuscript. Drs. Hingorani and Askenazi provided oversight and made substantial contributions to the design of this study and acquisition of data, reviewed, and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
All authors report no real or perceived conflicts of interest that could affect the study design; collection, analysis and interpretation of data; the writing of the report; or the decision to submit the manuscript for publication. For full disclosure, we provide the additional list of authors’ other commitments and funding sources that are not directly related to this study. R.H.S. receives grant funding for studies not related to this project from NHLBI and PCORI. S.L.G. reports personal fees from and a position as a consultant to Nuwellis, Renibus, ExThera, Reata and Medtronic Inc. S.L.G. has an equity interest in MediBeacon, Inc. S.L.G. receives grant funding from and serves as a consultant and on a Speaker’s Bureau for Baxter Healthcare, Inc. S.L.G. receives grant funding and serves as a consultant for BioPorto Diagnostics, Inc. S.L.G. serves on a Speaker’s Bureau for Fresenius Medical Corporation. D.J.A. consults for Baxter, Nuwellis, Medtronic, Bioporto and Seastar Medical. He also receives grant funding for studies not related to this project from Baxter, Nuwellis, Medtronic, SeaStar Medical, Bioporto, and National Institutes of Health (R01 FD005092, U34 KD117128). He is founder and Chief Scientific Officer for Zorro-Flow Inc. He has patents pending for urine collection device and System for Continuous Renal Replacement System.
Consent to participate
Caregiver consent was required for participation in primary (PENUT) study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Starr, M.C., Schmicker, R.H., Halloran, B.A. et al. Premature infants born <28 weeks with acute kidney injury have increased bronchopulmonary dysplasia rates. Pediatr Res 94, 676–682 (2023). https://doi.org/10.1038/s41390-023-02514-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02514-4
This article is cited by
-
Low incidence of acute kidney injury in VLBW infants with restrictive use of mechanical ventilation
Pediatric Nephrology (2024)
-
Blood pressure in preterm infants with bronchopulmonary dysplasia in the first three months of life
Pediatric Nephrology (2024)
-
Acute kidney injury decreases pulmonary vascular growth and alveolarization in neonatal rat pups
Pediatric Research (2023)