Abstract
Objectives
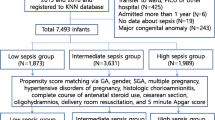
This study aims to develop a predictive model to assess the probability of poor prognosis in very low birth weight infants (VLBWI) with late-onset sepsis (LOS).
Methods
A total of 309 eligible VLBWI with LOS were included in the study. Logistic regression was used to determine prognostic factors for VLBWI with LOS. A nomogram incorporating these factors was created to predict the probability of poor prognosis. Poor prognosis includes death and survival with severe complications.
Results
In the developmental cohort, the incidence of poor prognosis was 59.5% (147/247). Forward stepwise logistic regression analysis showed that HCO3, albumin (ALB), ionized calcium (iCa), blood urea nitrogen (BUN), gestational age (GA), and birth weight (BW) were independent predictors of poor prognosis in VLBWI with LOS. The predictive model showed good discrimination and calibration. In the developmental cohort, the prediction model had a sensitivity of 83.7%, a specificity of 74.0%, and a C-index of 0.845 (95% confidence interval: 0.795–0.894).
Conclusion
Our study identified independent predictors of poor prognosis in VLBWI with LOS and used them to construct a predictive model. This model can help clinicians to identify high-risk groups with poor prognosis early and provide important clinical reference information.
Impact
This article highlights the development of a predictive model to assess the probability of poor prognosis in very low birth weight infants with late-onset sepsis (LOS). The model constructed in this manuscript was the first model to predict the poor prognosis of VLBWI with LOS. We mean a poor prognosis that includes death and some severe complications that may lead to long-term disability. Clinicians can use the model’s scoring results to assess a patient’s condition and accurately identify the occurrence of poor prognosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Liu, L. et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 385, 430–440 (2015).
Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128 (2012).
Shane, A. L., Sánchez, P. J. & Stoll, B. J. Neonatal sepsis. Lancet 390, 1770–1780 (2017).
Gu, M., Mei, X. L. & Zhao, Y. N. Sepsis and cerebral dysfunction: bbb damage, neuroinflammation, oxidative stress, apoptosis and autophagy as key mediators and the potential therapeutic approaches. Neurotox. Res. 39, 489–503 (2021).
Ebrahimi, M. E. et al. The association between clinical and biochemical characteristics of late-onset sepsis and bronchopulmonary dysplasia in preterm infants. Eur. J. Pediatr. 180, 2147–2154 (2021).
Wang, X., Tang, K., Chen, L., Cheng, S. & Xu, H. Association between sepsis and retinopathy of prematurity: a systematic review and meta-analysis. BMJ Open 9, 2018–025440 (2019).
Del Pozo, J. L. Stewardship in sepsis. Rev. Esp. Quimioter. 2, 42–46 (2019).
van Engelen, T. S. R., Wiersinga, W. J., Scicluna, B. P. & van der Poll, T. Biomarkers in Sepsis. Crit. Care Clin. 34, 139–152 (2018).
Lippi, G. Sepsis biomarkers: past, present and future. Clin. Chem. Lab. Med. 57, 1281–1283 (2019).
Marlow, N., Wolke, D., Bracewell, M. A. & Samara, M. Neurologic and developmental disability at six years of age after extremely preterm birth. N. Engl. J. Med 352, 9–19 (2005).
van Haastert, I. C. et al. Decreasing incidence and severity of cerebral palsy in prematurely born children. J. Pediatr. 159, 86–91.e81 (2011).
Pascal, A. et al. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: a meta-analytic review. Dev. Med Child Neurol. 60, 342–355 (2018).
Sun, W. et al. A nomogram for predicting the in-hospital mortality after large hemispheric infarction. BMC Neurol. 19, 347 (2019).
Zhang, W. et al. Development and validation of a CT-based radiomic nomogram for preoperative prediction of early recurrence in advanced gastric cancer. Radiother. Oncol. 145, 13–20 (2020).
Sun, J., Zhang, Z. & OuYang, J. A novel nomogram combined PIRADS v2 and neutrophil-to-lymphocyte ratio to predict the risk of clinically significant prostate cancer in men with PSA < 10 ng/ml at first biopsy. Urol. Oncol. 38, 401–409 (2020).
Ge, W. J. et al. Prediction of neonatal outcomes in extremely preterm neonates. Pediatrics 132, e876–e885 (2013).
Mercier, C. E., Dunn, M. S., Ferrelli, K. R., Howard, D. B. & Soll, R. F. Neurodevelopmental Outcome of Extremely Low Birth Weight Infants from the Vermont Oxford Network: 1998–2003. Neonatology 97, 329–338 (2010).
Hamrick, S. E. et al. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: the role of cystic periventricular leukomalacia. J. Pediatr. 145, 593–599 (2004).
Schmidt, B. et al. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA 289, 1124–1129 (2003).
Rees, C. M., Pierro, A. & Eaton, S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch. Dis. Child Fetal Neonatal Ed. 92, F193–F198 (2007).
Bhandari, A. & Bhandari, V. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics 123, 1562–1573 (2009).
Jeng, S. F. et al. Bronchopulmonary dysplasia predicts adverse developmental and clinical outcomes in very-low-birthweight infants. Dev. Med Child Neurol. 50, 51–57 (2008).
Vrijlandt, E. J. L. E., Boezen, H. M., Gerritsen, J., Stremmelaar, E. F. & Duiverman, E. J. Respiratory health in prematurely born preschool children with and without bronchopulmonary dysplasia. J. Pediatr. 150, 256–261 (2007).
Higgins, R. D. et al. Bronchopulmonary dysplasia: executive summary of a workshop. J. Pediatr. 197, 300–308 (2018).
Wynn, J. L. Defining neonatal sepsis. Curr. Opin. Pediatr. 28, 135–140 (2016).
Huang, J. et al. Cumulative evidence for association of sepsis and retinopathy of prematurity. Medicine 98, 0000000000017512 (2019).
Villamor-Martinez, E. et al. Cerebellar Hemorrhage in Preterm Infants: A Meta-Analysis on Risk Factors and Neurodevelopmental Outcome. Front Physiol. 10, 800 (2019).
Chew, M. S. et al. Increased plasma levels of heparin-binding protein in patients with shock: a prospective, cohort study. Inflamm. Res 61, 375–379 (2012).
Han, D. et al. Prognostic value of blood urea nitrogen/creatinine ratio for septic shock: an analysis of the MIMIC-III clinical database. Biomed. Res. Int. 2021, 5595042 (2021).
Li, T. et al. Predictive value of c-reactive protein-to-albumin ratio for neonatal sepsis. J. Inflamm. Res 14, 3207–3215 (2021).
Li, X. et al. Higher blood urea nitrogen level is independently linked with the presence and severity of neonatal sepsis. Ann. Med 53, 2192–2198 (2021).
Jat, K. R., Jhamb, U. & Gupta, V. K. Serum lactate levels as the predictor of outcome in pediatric septic shock. Indian J. Crit. Care Med. 15, 102–107 (2011).
Low, J. A., Lindsay, B. G. & Derrick, E. J. Threshold of metabolic acidosis associated with newborn complications. Am. J. Obstet. Gynecol. 177, 1391–1394 (1997).
Fee, S. C., Malee, K., Deddish, R., Minogue, J. P. & Socol, M. L. Severe acidosis and subsequent neurologic status. Am. J. Obstet. Gynecol. 162, 802–806 (1990).
Jonsson, M., Agren, J., Norden-Lindeberg, S., Ohlin, A. & Hanson, U. Suboptimal care and metabolic acidemia is associated with neonatal encephalopathy but not with neonatal seizures alone: a population-based clinical audit. Acta Obstet. Gynecol. Scand. 93, 477–482 (2014).
Fanali, G. et al. Human serum albumin: from bench to bedside. Mol. Asp. Med 33, 209–290 (2012).
Artigas, A., Wernerman, J., Arroyo, V., Vincent, J. L. & Levy, M. Role of albumin in diseases associated with severe systemic inflammation: Pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J. Crit. Care 33, 62–70 (2016).
Soeters, P. B., Wolfe, R. R. & Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. JPEN J. Parenter. Enter. Nutr. 43, 181–193 (2019).
Don, B. R. & Kaysen, G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 17, 432–437 (2004).
Arroyo, V., Garcia-Martinez, R. & Salvatella, X. Human serum albumin, systemic inflammation, and cirrhosis. J. Hepatol. 61, 396–407 (2014).
Eckart, A. et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am. J. Med 133, 713–722.e717 (2020).
Sheinenzon, A., Shehadeh, M., Michelis, R., Shaoul, E. & Ronen, O. Serum albumin levels and inflammation. Int J. Biol. Macromol. 184, 857–862 (2021).
Takegawa, R. et al. Serum albumin as a risk factor for death in patients with prolonged sepsis: An observational study. J. Crit. Care 51, 139–144 (2019).
Kendall, H., Abreu, E. & Cheng, A. L. Serum albumin trend is a predictor of mortality in ICU patients with sepsis. Biol. Res. Nurs. 21, 237–244 (2019).
Yin, M. et al. Predictive value of serum albumin level for the prognosis of severe sepsis without exogenous human albumin administration: a prospective cohort study. J. Intensive Care Med. 33, 687–694 (2018).
Yang, C. et al. Relationship between serum albumin levels and infections in newborn late preterm infants. Med Sci. Monit. 22, 92–98 (2016).
Brun-Buisson, C. et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA 274, 968–974 (1995).
Ballmer-Weber, B. K., Dummer, R., Küng, E., Burg, G. & Ballmer, P. E. Interleukin 2-induced increase of vascular permeability without decrease of the intravascular albumin pool. Br. J. Cancer 71, 78–82 (1995).
Doweiko, J. P. & Nompleggi, D. J. The role of albumin in human physiology and pathophysiology, Part III: Albumin and disease states. JPEN J. Parenter. Enter. Nutr. 15, 476–483 (1991).
Nesseler, N. et al. Clinical review: The liver in sepsis. Crit Care. 16, 235 https://doi.org/10.1186/cc11381 (2012).
Sitar, M. E., Aydin, S. & Cakatay, U. Human serum albumin and its relation with oxidative stress. Clin. Lab 59, 945–952 (2013).
Fleck, A. et al. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet 1, 781–784 (1985).
Kelly, A. & Levine, M. A. Hypocalcemia in the critically ill patient. J. Intensive Care Med. 28, 166–177 (2013).
Liu, Y., Chai, Y., Rong, Z. & Chen, Y. Prognostic value of ionized calcium levels in neonatal sepsis. Ann. Nutr. Metab. 76, 193–200 (2020).
Hu, H. et al. A prediction model for assessing prognosis in critically Ill patients with sepsis-associated acute kidney injury. Shock 56, 564–572 (2021).
Tóth-Heyn, P., Drukker, A. & Guignard, J. P. The stressed neonatal kidney: from pathophysiology to clinical management of neonatal vasomotor nephropathy. Pediatr. Nephrol. 14, 227–239 (2000).
Nusshag, C., Weigand, M. A., Zeier, M., Morath, C. & Brenner, T. Issues of acute kidney injury staging and management in sepsis and critical illness: a narrative review. Int J. Mol. Sci. 18, 1387 (2017).
Kibria, G. M. A. et al. Determinants of early neonatal mortality in Afghanistan: an analysis of the Demographic and Health Survey 2015. Glob. Health 14, 47 (2018).
Alshaikh, B., Yusuf, K. & Sauve, R. Neurodevelopmental outcomes of very low birth weight infants with neonatal sepsis: systematic review and meta-analysis. J. Perinatol. 33, 558–564 (2013).
Huang, Y. et al. Development and validation of a nomogram for predicting late-onset sepsis in preterm infants on the basis of thyroid function and other risk factors: Mixed retrospective and prospective cohort study. J. Adv. Res. 24, 43–51 (2020).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. LLW conceived and supervised the study; XJZ, JYC, QYC were involved in the data collection and analysis; The first draft of the manuscript was written by XJZ, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Our study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University. All manipulations involving humans in this study were under the principles in the Declaration of Helsinki. We confirm that all data are anonymous and confidential. Therefore, due to the retrospective nature of the current study, the requirement for informed consent has been waived.
Consent statement
This was a retrospective study, so the requirement for informed consent was waived.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, X., Chen, J., Cheng, Q. et al. A predictive model for prognosis in very low birth weight infants with late-onset sepsis. Pediatr Res 94, 643–652 (2023). https://doi.org/10.1038/s41390-023-02480-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02480-x