Abstract
Background
Neonatal hypoglycaemia can lead to brain damage and neurocognitive impairment. Neonatal hypoglycaemia is associated with smaller caudate volume in the mid-childhood. We investigated the relationship between neurodevelopmental outcomes and caudate volume and whether this relationship was influenced by neonatal hypoglycaemia.
Methods
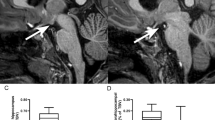
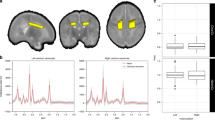
Children born at risk of neonatal hypoglycaemia ≥36 weeks’ gestation who participated in a prospective cohort study underwent neurodevelopmental assessment (executive function, academic achievement, and emotional-behavioural regulation) and MRI at age 9–10 years. Neonatal hypoglycaemia was defined as at least one hypoglycaemic episode (blood glucose concentration <2.6 mmol/L or at least 10 min of interstitial glucose concentrations <2.6 mmol/L). Caudate volume was computed using FreeSurfer.
Results
There were 101 children with MRI and neurodevelopmental data available, of whom 70 had experienced neonatal hypoglycaemia. Smaller caudate volume was associated with greater parent-reported emotional and behavioural difficulties, and poorer prosocial behaviour. Caudate volume was significantly associated with visual memory only in children who had not experienced neonatal hypoglycaemia (interaction p = 0.03), but there were no other significant interactions between caudate volume and neonatal hypoglycaemia.
Conclusion
Smaller caudate volume is associated with emotional behaviour difficulties in the mid-childhood. Although neonatal hypoglycaemia is associated with smaller caudate volume, this appears not to contribute to clinically relevant neurodevelopmental deficits.
Impact
-
At 9–10 years of age, caudate volume was inversely associated with emotional-behavioural difficulties and positively associated with prosocial behaviour but was not related to executive function or educational achievement.
-
Previous studies have suggested that neonatal hypoglycaemia may contribute to smaller caudate volume but exposure to neonatal hypoglycaemia did not appear to influence the relationship between caudate volume and behaviour.
-
Among children not exposed to neonatal hypoglycaemia, caudate volume was also positively associated with visual memory, but no such association was detected among those exposed to neonatal hypoglycaemia.
-
Understanding early-life factors that affect caudate development may provide targets for improving behavioural function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Published data are available to approved researchers under the data sharing arrangements provided by the Clinical Data Research Hub, based at the Liggins Institute, University of Auckland (https://wiki.auckland.ac.nz/researchhub). Metadata, along with instructions for data access, are available at the University of Auckland’s research data repository, Figshare (https://auckland.figshare.com). Data access requests are to be submitted to Data Access Committee via researchhub@auckland.ac.nz. Deidentified published data will be shared with researchers who provide a methodologically sound proposal and have appropriate ethical and institutional approval. Researchers must sign and adhere to the Data Access Agreement that includes a commitment to using the data only for the specified proposal, to refrain from any attempt to identify individual participants, to store data securely, and to destroy or return the data after completion of the project. The Clinical Data Research Hub reserves the right to charge a fee to cover the costs of making data available, if required.
References
Harding, J. E., Harris, D. L., Hegarty, J. E., Alsweiler, J. M. & McKinlay, C. J. An emerging evidence base for the management of neonatal hypoglycaemia. Early Hum. Dev. 104, 51–56 (2017).
Alkalay, A. L., Flores-Sarnat, L., Sarnat, H. B., Moser, F. G. & Simmons, C. F. Brain imaging findings in neonatal hypoglycemia: case report and review of 23 cases. Clin. Pediatr. (Philos.). 44, 783–790 (2005).
Shah, R., Harding, J., Brown, J. & McKinlay, C. Neonatal glycaemia and neurodevelopmental outcomes: a systematic review and meta-analysis. Neonatology 115, 116–126 (2019).
Filan, P. M., Inder, T. E., Cameron, F. J., Kean, M. J. & Hunt, R. W. Neonatal hypoglycemia and occipital cerebral injury. J. Pediatr. 148, 552–555 (2006).
Burns, C. M., Rutherford, M. A., Boardman, J. P. & Cowan, F. M. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics 122, 65–74 (2008).
Shah, R. et al. Association of neonatal hypoglycemia with academic performance in mid-childhood. JAMA 327, 1158 (2022).
McKinlay, C. J. D. et al. Association of neonatal glycemia with neurodevelopmental outcomes at 4.5 years. JAMA Pediatr. 171, 972–983 (2017).
Nivins, S. et al. Associations between neonatal hypoglycaemia and brain volumes, cortical thickness and white matter microstructure in mid-childhood: an MRI study. Neuroimage Clin. 33, 102943 (2022).
Grahn, J. A., Parkinson, J. A. & Owen, A. M. The cognitive functions of the caudate nucleus. Prog. Neurobiol. 86, 141–155 (2008).
Graff-Radford, J., Williams, L., Jones, D. T. & Benarroch, E. E. Caudate nucleus as a component of networks controlling behavior. Neurology 89, 2192 (2017).
Abernethy, L. J., Cooke, R. W. I. & Foulder-Hughes, L. Caudate and hippocampal volumes, intelligence, and motor impairment in 7-year-old children who were born preterm. Pediatr. Res. 55, 884–893 (2004).
Fryer, S. L. et al. Caudate volume predicts neurocognitive performance in youth with heavy prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 36, 1932–1941 (2012).
McKinlay, C. J. D. et al. Neonatal glycemia and neurodevelopmental outcomes at 2 years. N. Engl. J. Med. 373, 1507–1518 (2015).
Harris, D. L., Weston, P. J., Signal, M., Chase, J. G. & Harding, J. E. Dextrose gel for neonatal hypoglycaemia (the Sugar Babies Study): a randomised, double-blind, placebo-controlled trial. Lancet 382, 2077–2083 (2013).
Harris, D. L., Battin, M. R., Weston, P. J. & Harding, J. E. Continuous glucose monitoring in newborn babies at risk of hypoglycemia. J. Pediatr. 157, 198–202 (2010).
Dale, A. M., Fischl, B. & Sereno, M. I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9, 179–194 (1999).
Fischl, B., Liu, A. & Dale, A. M. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging 20, 70–80 (2001).
Fischl, B. et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage 23, 69–84 (2004).
Minister of Education. e-asTTle. http://e-asttle.tki.org.nz/. Accessed 15 Sept 2016.
Goodman, R. The Strengths and Difficulties Questionnaire: a research note. J. Child Psychol. Psychiatry 38, 581–586 (1997).
Robinson, J. L. et al. The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage 60, 117–129 (2012).
Haber, S. N. Neuroanatomy of reward: a view from the ventral striatum. Neurobiol. Sensat. Reward 255–282 https://doi.org/10.1201/b10776-19 (2011).
Crane, L., Sumner, E. & Hill, E. L. Emotional and behavioural problems in children with Developmental Coordination Disorder: exploring parent and teacher reports. Res. Dev. Disabil. 70, 67–74 (2017).
Gritti, A. et al. Epidemiological study on behavioural and emotional problems in developmental age: prevalence in a sample of Italian children, based on parent and teacher reports. Ital. J. Pediatr. 40, 19 (2014).
Tremols, V. et al. Differential abnormalities of the head and body of the caudate nucleus in attention deficit-hyperactivity disorder. Psychiatry Res. 163, 270–278 (2008).
Acknowledgements
We are grateful to the children and families who participated in this study. We thank the staff of the Centre for Advanced Magnetic Resonance Imaging and Midland Radiology for MRI scanning and the Centre for eResearch for computing support. This research was supported by use of the Nectar Research Cloud, a collaborative Australian research platform supported by the NCRIS-funded Australian Research Data Commons (ARDC). We would like to thank the CHYLD steering group for their input.
Funding
Health Research Council of New Zealand (17/240); Neurological Foundation (1729PG), and Liggins Institute PhD scholarship (awarded to S.N.).
Author information
Authors and Affiliations
Consortia
Contributions
E.K.: conceptualization, formal analysis, resources, writing—original draft. S.N.: investigation, writing—review and editing. B.T.: funding acquisition, writing—review and editing. C.J.D.M.: conceptualization, funding acquisition, writing—review and editing. J.H.: conceptualization, supervision, funding acquisition, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
Parents or primary caregivers provided written informed consent and the child provided assent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kennedy, E., Nivins, S., Thompson, B. et al. Neurodevelopmental correlates of caudate volume in children born at risk of neonatal hypoglycaemia. Pediatr Res 93, 1634–1641 (2023). https://doi.org/10.1038/s41390-022-02410-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02410-3