Abstract
Background
There is a need for further understanding pediatric long COVID syndrome (LCS) to be able to create specific case definitions and guidelines for providing good clinical care.
Methods
Medical records of all LCS patients who presented at our designated LC clinic were collected. We carried out descriptive analyses summarizing the history, clinical presentation, and findings of children, while doing a diagnosis of exclusion with multi-disciplinary medical examinations (physical, laboratory, and radiological examinations, specialist consultations, etc.) without a control group.
Results
Most children reported at least minor impairment to their quality of life, of which 17 (23%) had moderate or severe difficulties. Findings that could be directly connected to the linked complaint category were observed in an average of 18%, respiratory symptoms with objective alterations being the most frequent (37%). Despite our detecting mostly non-specific conditions, in a smaller number we identified well-described causes such as autoimmune thyroiditis (7%).
Conclusions
The majority of children stated an impairment in their quality of life, while symptom-related conditions were detected only in a minority. Controlled studies are needed to separate the effect of the pandemic era from the infection itself. Evidence-based pediatric guidelines could aid to rationalize the list of recommended examinations.
Impact
-
Long COVID syndrome is a complex entity with a great impact on children’s everyday lives. Still, there is no clear guidance for pediatric clinical management. Systematic, detailed studies with medical assessment findings could aid the process of creating evidence-based guidelines.
-
We present validated systematic information collected during in-person medical assessments with detailed medical findings and quality of life changes.
-
While making a diagnosis of exclusion, we could confirm symptom-related conditions only in a minority of children; however, the majority reported at least minor impairment to their quality of life.
Similar content being viewed by others
Introduction
Based on the considerable research conducted in the past 2 years, children have been indicated to have relatively lower rates of severe acute Coronavirus disease 2019 (COVID-19).1 However, the latest clinical experience shows that post-acute consequences of the infection could mean a risk of similarly impactful outcomes.2,3
The World Health Organization (WHO) developed a clinical case definition of post-COVID condition by Delphi consensus in October 2021; however, it is indicated that it might not be suitable for children.4 Estimating the epidemiological and medical characteristics of COVID-19-related diseases in children is difficult. Based on a meta-analysis published in August 2021, it is predicted that 35% of all cases remained asymptomatic throughout the infection.5 According to a recent British calculation, the prevalence of symptoms that remain 12 weeks after Coronavirus infection ranges from 3% based on tracking specific symptoms to 12% based on self-classification of long COVID syndrome (LCS; https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/technicalarticleupdatedestimatesoftheprevalenceofpostacutesymptomsamongpeoplewithcoronaviruscovid19intheuk/26april2020to1august2021).
LCS is a growing health concern as its persisting and emerging symptoms are extensive, often overlapping, fluctuating, changing over time, and can affect multiple organ systems with a potentially negative impact on the quality of life.6,7 In the pediatric field, only a few prospective studies collecting systematic data in larger cohorts of children with multidisciplinary clinical assessment are available. They also often fail to include quality of life changes, detailed clinical characteristics, or confirmation of the acute infection.8 Other studies that followed up patients previously hospitalized at the acute phase of COVID are reporting answers given to interviews without clinical assessment.9 Studies focusing on the long-lasting neurological,10 cardiac,11 pulmonary,12 or psychological13 effects of COVID-19 infection are available, but only separately.
In February 2021, an increasing number of children presented at our Emergency Unit at the 1st Department of Pediatrics, Semmelweis University with permanent, often debilitating symptoms. We opened our LC outpatient clinic in March 2021 with the purpose of further investigating this condition and understanding the natural history of the disease.
Since the course of the illness seems to be different in children than in adults,14 we aim in our present case series to provide a deeper insight into pediatric LCS.
Methods
Data collection
In our observational study, patients underwent standard-of-care examinations, and blood samples were collected primarily for diagnostic purposes. Data was collected as a component of ambulatory care and stored in a standardized case report form (CRF), which included online surveys for patients and employed the WHO Post COVID CRF (https://www.who.int/publications/i/item/global-covid-19-clinical-platform-case-report-form-(crf)-for-post-covid-conditions-(post-covid-19-crf-)) for data collection. It consists of questions about the acute infection, lingering symptoms, and functioning and medical findings, which we extended and translated to match the institute-specific purposes. Responses were validated and additional outcomes were recorded by the physician in the CRF. We have been granted an ETT TUKEB ethical approval, the number of which is: IV/5943-1/2021/EKU.
Case definition
Utilizing the National Institute for Clinical Excellence (NICE) guideline’s case definitions,15 LCS cases were defined based on the presence of specific symptoms and the clinical (for example, olfactory impairment) and/or laboratory evidence of COVID-19 at least 1 month preceding the current symptoms.
The guideline distinguishes three categories related to COVID-19; first, the “acute disease,” which is present for up to 4 weeks from first symptom onset. The “ongoing symptomatic” phase, which can be used for conditions reported after the acute infection, but not lasting longer than 12 weeks; and “post-COVID-19 syndrome,” which refers to symptoms and signs occurring since or after an infection related to COVID-19 continue for more than 12 weeks and are not explained by an alternative diagnosis. Symptoms (usually clusters of symptoms) can overlap, fluctuate, and change over time and can affect any organ system.15
The term “long COVID,” which we prefer to use, until the presence of a specific clinical case definition for children, includes both the “ongoing symptomatic” phase and “post-COVID syndrome.”15 To present as reliable data as possible, we excluded those from our analysis who met the criteria of the NICE case definition with a strong clinical suspicion of the acute infection but for whom we could not confirm the acute COVID with laboratory tests.
Study sample and patient management
Data were collected during the visits undertaken at the 1st Department of Pediatrics from March 24 through May 26, 2021. Patients could make online appointments to our facility from across the country without restrictions. A general practitioner’s referral or a preassessment was not required. Before their first visit, parents filled out the online questionnaire with the help of their children regarding the general, acute COVID-19-specific, and LCS-specific history and persisting symptoms, as well as the quality of the children’s lives. The survey contained a total of 50 questions about their current complaints, which could be categorized into 10 major organ system categories (general, cardiovascular, neurologic, mental, gastrointestinal [GI], dermatologic, musculoskeletal, pulmonary, ear–nose–throat and ophthalmologic [ENT-O], and reproductive) as seen in Supplementary Table S1.
A Quality of Life (Functioning) (QoL-F) component was also included in the questionnaire, which was measured by 12 items on a 5-grade scale from “no difficulty” to “extreme difficulty/cannot do.” Furthermore, the QoL-F items were compared to the situation before COVID-19 on a 3-point scale (better, same, worse) to measure changes in QoL-F. The total scores of the QoL-F and QoL-F changes were converted to an index value between 0 and 100, taking into account the number of answered questions (QoL-F: 0—no difficulty, 100—extreme difficulty/cannot do; QoL-F changes: 0—better, 50—same, 100—worse).
Relying on the NICE guideline,16 all patients underwent physical examination and blood tests, with particular emphasis on the following parameters: complete blood count, clinical chemistry tests [ions, liver function (GOT, GPT, GGT, ALP), renal function (urea, creatinine), CRP, CK, protein, albumin, LDH, ferritin], IL-6, BNP, D-dimer, troponin, thyroid function (TSH, T3, T4), coagulation parameters, and autoantibodies specific for autoimmune thyroiditis (ATG, ATPO, TRAK) and COVID-19 IgG, IgM antibodies.
Further examinations (e.g., imaging) and consultations were indicated according to the symptoms and to the laboratory and physical findings.
Standardized study definitions
Normal levels of laboratory values were defined by institutional age-specific pediatric standards. The presence of a medical condition was based on discharge diagnoses in previous medical documentation or discharge diagnoses given during our checkup. Obesity was determined based on national age-specific body mass index Z scores.16
The list and details of undertaken examination can be found in Tables 1–3 and Supplementary Tables S4–S6.
Data extraction and statistical analysis
Data was extracted from the CRFs, stored in data tables, and re-validated before being used for data analysis with the help of professional biostatisticians. To describe the data, we used the distribution of relative frequencies and descriptive analysis. If not otherwise stated, data was presented as mean ± SD or absolute frequency and proportion. We used independent samples t test to examine sex differences with Hedges’ g effect size, confidence intervals, and Pearson’s correlation for associations between variables. The level of significance was set a priori at 0.05. Statistical analysis and visualization were conducted using IBM SPSS Statistics software, Version 25.0 (IBM Corp.), and qgraph package (version 1.6.9.) in R.17
Results
Demographics and SARS-COV-2 testing
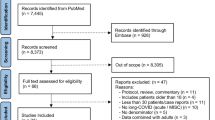
From March 24 through May 26, 2021, a total of 103 children sought ambulatory care at our LCS clinic; of these, 6 did not fulfill the LCS criteria.15 We excluded another 8 children from the analysis, who clinically fulfilled the case definition, but we could not verify the presence of the acute infection, therefore we enrolled 89 in our final analysis.
Of the 89 patients with LCS, 33 (37.1%) were male. The mean age was 11.4 ± 3.6 years, lower in males than in females (10.1 vs. 12.3 years, P = 0.02, g = 0.60 [0.16; 1.03]). All patients belonged to the Caucasian ethnicity (Table 4).
Before their first visit to our clinic, 83 (93%) children were screened for laboratory evidence of COVID-19, and 80 (83%) tested positive. Those children who did not have a confirmed diagnosis before, 9 tested positive for SARS-CoV-2 IgG antibodies at our clinic, leading to a total of 89 (100%) with a confirmed SARS-CoV-2 infection (for further details, see Supplementary Table S2).
Overall, 84 (94%) children had a mild or asymptomatic acute COVID-19 illness, while 5 had a moderate acute disease based on the WHO’s classification (https://www.who.int/publications/i/item/global-covid-19-clinical-platform-case-report-form-(crf)-for-post-covid-conditions-(post-covid-19-crf-)). Details of symptoms, the needed medical care during acute COVID and details of medical history can be found in Supplementary Table S3. None of the patients had been vaccinated for SARS-CoV-2 at the time of their first visit.
Persisting symptoms
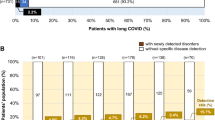
The average number of symptoms per individual was 12 (ranging from 1 to 33), with the leading complaint—persistent fatigue—occurring in 70% of the children (Fig. 1 and Table 4). Of the 10 organ system categories, an average of 5 ± 2 were affected per person. Similar to acute COVID, general and neurologic complaints were dominating, though mental and cardiologic complaints appeared more frequently in LCS than in acute infection (Fig. 2a and Table 4). The frequently occurring symptoms also showed a tendency to co-occur (Fig. 2b).
a Comparing the frequency of symptoms categorized by organ systems during and after acute COVID. *The questionnaire regarding acute symptoms did not contain questions about the reproductive system. b Symptoms network graph: the connection lines represent the frequencies of two variables presented simultaneously. gen general, neu nervous, psy mental, ent&o ENT-O (ear, nose, throat, and eye), pul pulmonary, car cardiovascular, der dermatologic, mus musculoskeletal, rep reproductive, gis gastrointestinal.
At the time of the first visit, symptoms had been present for an average duration of 17.8 weeks (ranging from 5 to 37 weeks); age and duration of symptoms showed a significant positive correlation (r(87) = 0.58 [0.42; 0.7], P < 0.001). In 58 (65%) children, at least one symptom was continuously present during and after the acute COVID sequel, and in only 9 cases the LCS-related symptoms arose more than a month after the acute disease. In 73 children, there was at least one new, intense symptom that the patient had never experienced before their acute infection; 10 children had symptoms that worsened in their intensity and/or quality, and 6 patients gave uncertain information about their complaints (Table 4).
Physical and specific findings, laboratory markers
During the physical examination, 45 (51%) children had alterations in their status. Mostly minor findings were observed, mild abdominal tenderness being the most frequent (Supplementary Table S4).
Laboratory examinations showed alterations mostly of low clinical importance or that were hard to interpret because of the lack of other supporting findings (Table 2). However, blood testing alone led to the suspicion of 10 (12%) autoimmune-thyroiditis with specific autoantibodies detected, 4 of whom also had elevated thyroid-stimulating hormone (TSH) values. We diagnosed thyroiditis in 7% of all cases based on the recognition of elevated autoimmune antibody levels followed by positive ultrasound results. (Tables 2 and 3 and Supplementary Table S5).
We indicated at least one imaging examination for 71 children and found alterations in 28% (Table 3 and Supplementary Table S5).
We performed a complete 6-min walk test in 29 cases and more than half (20/29, 67%) of patients reported some complaints during the test, although only 10 (34%) had unquestionable difficulties during the exercise (had to stop, had a drop in SpO2 < 95%, or heart rate elevated to 1.5× of their resting heart rate) (Supplementary Tables S5 and S6).
In the 74 children with neurologic complaints, neurologic examinations had non-physiological results in only 7% (5/74) but also revealed a case of trigeminal cephalalgia with polyneuropathy. Overall, 28% of children had hypo/dysosmia with hypo/dysgeusia, and 4% had hypo/dysosmia alone. Seventy-two percent of the children reported having mental problems at some point during their disease; a consultation was necessary in 24 cases, resulting in 15 (23%) new preliminary diagnoses, including major depression and anxiety. Of the 55 children with cardiac complaints, 44 had cardiology-directed examinations of which only 15% (8/55) gave a positive result, though one case of recent myocarditis and one of long-QT syndrome were revealed. Respiratory complaints occurred in 30 children of whom 11 had positive results (37%). Complaints of the GI tract were reported in 53 children, and we indicated 37 GI-oriented examinations and found alterations in 15% (8/53). Dermatologic complaints occurred in 23 children at some point during their disease, and we indicated 8 consultations with dermatologists leading to 5 novel findings, including one Henoch–Schönlein syndrome.
On average, in 25% of the cases we were able to detect at least minor clinical alterations. (Table 1). We detected physical findings that could be directly connected to the linked complaint category in an average of 18% per category. We found the greatest proportion (38%) of connected physical alterations with the specific complaint in the respiratory category (Table 1).
Additional information on our specific findings is available in Supplementary Tables S4 and 5.
Treatment
As there is no specific treatment available for LCS, in most cases we could only give non-specific supportive therapy for most symptoms. Within the framework of complex, individualized rehabilitation, we offered NSAID for pain, physiotherapy for musculoskeletal problems, Transcutaneous Electrical Nerve Stimulation (TENS) for tension headache, breathing exercises for respiratory disturbances. We also highlighted the beneficial effects of having a scheduled daily routine with regular physical activity and relaxation.
We suggested performing a 12 week long smell training with different types of fragrance oils of choice practiced two times daily for patients with dysosmia or anosmia. In such cases, we frequently observed the complete or partial refusal of meat or other essential nutrient consumption. A short dietary advice handbook was created by a professional dietitian for whom the execution of a healthy, balanced diet seemed to be problematic. It lists multiple alternative nutrient sources, suggests web based and mobile applications for exploring different cooking techniques and approaches which could help masking the disturbing elements of meals. To create a personalized meal plan, an in-person meeting was also available with the dietitian, particularly for those with weight loss. Initiation of transient formula feeding was necessary in two severe cases.
Children with definite diagnoses were treated according to pediatric protocols, as L-Thyroxin therapy was started in two children, inhaled corticosteroids were prescribed in 7 cases, and montelukast in one child.
Functioning
There was a total of 53 participants who completed the questions without missing data; we also included those who answered at least half of the questions (n = 14). The total score of QoL-F was between 0 and 90.9 (M = 27.1, SD = 22.8) which means mostly mild difficulties in functioning. The total score of QoL-F changes was between 45.8 and 100 (M = 70.4, SD = 16.3) meaning that deterioration was most commonly reported, compared to the period before COVID-19. Overall, 14 participants (26%) experienced both at least moderate (QoL-F ≥ 50) difficulties and a worsened status in functioning since COVID-19. The most frequently reported difficult and/or worsened functions were: being emotionally affected by health problems, walking a long distance, day-to-day school/work, standing for long periods, and concentrating for 10 min (Fig. 3a–c).
a The QoL-F of children, measured by the 12 items on a 5-grade scale. b The QoL-F of children compared to their situations before COVID-19 on a 3-point scale. c Association between the unbiased indexes of QoL-F (Difficulties in functioning) and QoL-F changes (Changes compared to pre-COVID-19 status).
We asked a separate question about the ability to self-care. Of the 73 who answered, 21 (29%) patients reported an impaired function, while 52 (71%) experienced no change.
Discussion
Our study involving 89 pediatric LCS patients from Hungary describes the clinical characteristics of a newly emerged, virus-associated chronic condition impairing children’s quality of life for months.18 Based on the NICE guideline’s clinical management guidance, we did a diagnosis of exclusion with multi-disciplinary medical examinations (physical, laboratory, radiological examinations, specialist consultations, etc.) without a control group.
We found their recommendation, i.e., to start screening in children as early as the at the “ongoing symptomatic” phase to sit well with our clinical experience, while in adults, it might be acceptable to start the clinical investigations only from the post-COVID phase (i.e., 3 months after).
Our patients reported a broad spectrum and severity of long-lasting symptoms, with fatigue, loss of interest, and headache being the most common ones. In a pediatric meta-analysis, the three most frequently reported symptoms were similar, except they found “dyspnea’ to be second. Based on five controlled studies, out of 14 investigated symptoms, the only ones with significant risk differences between previously COVID-positive and COVID-negative children were loss of smell, cognitive difficulties, sore eyes, sore throat, and headache.19
We observed both entirely new complaints in addition to the previously existing, chronic symptoms whose characteristics (intensity, frequency, and quality) worsened after the acute COVID-19. We found patients with exceedingly numerous complaints and with a duration of symptoms over 9 months at the time of our examination. Since LCS is a newly emerged condition, it is yet to be determined how long these effects can last. Based on 13 studies, the median duration of follow-up was 125 days (IQR = 99–231) after acute SARS-CoV-2 infection.19 Our data are similar, with a mean of 125 days (ranging from 37 to 259 days; Med = 130, IQR = 104.5 Q1–Q3 = 70.5–175).
Separating the symptoms of LCS from the ones that arose due to the pandemic situation can be challenging, as it is presumed that public restrictions themselves (e.g., lockdowns, online learning) could cause somatization.20 The observed high frequency of mental health complaints in our dataset could at least partly be an indicator of this phenomenon. Further clarification is required to determine which of these symptoms derive from the physiopathology of SARS-CoV-2 infection and which from the psychological effects of the disease burden, worsened by the restrictions of the pandemic.13
A notable controlled questionnaire study (CLoCk) showed that post-COVID-like symptoms also arose in the general pediatric population but not as many as in those who had COVID-19.21
With complex physical, laboratory, and further targeted examinations, we found a high number of minor abnormalities which had no or questionable clinical connection with COVID-19.
Most of our patients had similar neurologic symptoms as adults, like headache, memory problems, and/or smell or taste disturbances, however, in our cohort, one-third of children had hypo/dysosmia, which is much higher than Behnood et al. assumed.19
According to a recent meta-analysis, there is still no standard screening protocol for neurological complaints. For whom the neurological physical examination status proved to be seriously altered (blurred vision, diplopia, ptosis, etc.), we executed acute neuro-specific examinations. Both the two non-physiological head MRI and CSF results were of questionable clinical importance and required no specific therapy. We diagnosed one trigeminal cephalalgia with polyneuropathy with the typical complaints and changes in the neurological physical status.22 We offered her physiotherapy and clomipramine, but she refused to take the medicine. For the first three months in the follow-up period her complaints remained to persist. The need for further neurologic and psychiatric care were also communicated.
The observed number of neuro-psychiatric complaints (anxiety, depression, etc.) were also high in our dataset and were similar to those in adults and led to several preliminary diagnoses after consultations with psychologists.22 No psychotropic medication was prescribed.
Almost two-thirds of our patients had at least one cardiac (cardio-pulmonary) complaint, such as chest pain, palpitation, or post-exertional malaise. Adults experience similar persisting cardiac symptoms and in some cases, myocarditis, pericarditis, and cardiac fibrosis were detected.22 Our initial evaluations consisted of 6mWT, ECG, echocardiography, CRP-, troponin- and pro-BNP levels. Although, we could not find increased troponin levels in any of the children, in some cases, we observed slight elevations in the pro-BNP levels, however, with no clinical consequences. We diagnosed multiple post-viral tachycardias and in one case, the suspicion of a recent myocarditis was raised by echocardiography, however cardiac MRI could not confirm it.
Children with a mild acute disease usually do not develop severe pulmonary manifestations, thus we would not expect high rates of late complications with shortness of breath or persistent dry cough. Surprisingly, we observed respiratory complaints in around 34% of all children presenting in our facility with relatively high rates of positive findings with the 6mWT, chest X-Rays, and lung function tests. In adults, chest CT is a popular diagnostic tool for pulmonary consequences, as pulmonary fibrosis and ground-glass opacities can be observed frequently.22 Due to the effect of radiation, CT examination is restricted in the field of pediatrics. Still, at the beginning of our study, with the consultation of pediatric pulmonologists and radiologists, we performed three low-dose chest CTs, all with negative results. After reconsideration, we decided to further avoid these examinations. With the help of lung function tests, we diagnosed abnormalities with therapeutic consequences (inhaled corticosteroids) in seven cases.
Loss of appetite, abdominal pain, and nausea were the most commonly reported gastroenterological symptoms with mild abdominal tenderness being the most frequent physical examination finding among our patients. In most cases, lacking objective findings behind these GI complaints, we gave “post-viral abdominal pain” as a final diagnosis. However, we identified a few newly diagnosed cases of lactose intolerance, dysbiosis, or hepatic steatosis, neither of which we supposed to be in strong connection with the previous COVID-19.
The most frequently noted dermatologic complaints were rashes with a broad spectrum of presentation, extension, and color. We did not see any COVID toe23 or angioedema,22 but we examined a 3.5-year-old male who had been treated in an ICU 5 weeks before their visit with Henoch-Schönlein syndrome and COVID-19 infection, who was still on steroid therapy.
In our dataset, the ratio of symptom-related positive findings was low (a mean of 18% per complaint group), which could mean, that either the rate of somatization was high or that the presumed pathological effects of SARS-CoV-2 could not always be validated by currently available standard medical assessment techniques. Still, in a small but significant proportion of children, we could diagnose ultrasound-confirmed autoimmune thyroiditis, or obstructive respiratory disorders based on lung function test results. Until we know more about the long-term pathological effects of COVID-19, we need to perform a diagnosis of exclusion and cannot make a decision about a causative relationship. Adult case reports of Hashimoto’s thyroiditis and Graves’ disease24 after COVID-19 infection are present in the literature. It is known that viral infections can provoke or accelerate different autoimmune diseases and direct damage of SARS-CoV-2 through the ACE-2 receptor is hypothesized as well.25 This can also be true for all our outcomes, as SARS-CoV-2 can affect nearly all organ systems.6
Several mechanisms are hypothesized to lie behind LC symptoms, including viral cell damage, endothelial and microvascular damage, dysregulation of the immune system, hyperinflammation, hypercoagulational state with thrombosis, and the maladaptation of the ACE2 receptor.6 This pathomechanism may overlap with those of SARS and MERS virus; other opinions say that there is a resemblance with the post-Ebola or post-Chikungunya symptoms.26
Until LCS stays a diagnosis of exclusion, due to the complexity of symptoms, the length of examinations can be overwhelming for patients and their families both mentally and physically. Evidence-based pediatric guidelines built on controlled studies could aid to rationalize the list of recommended examinations.
Considering changes in the quality of life of children with LC, we mainly detected a long-lasting mild decline after the infection, although outstanding impairments were also observed. It should be noted that this deterioration in QoL scores and many of these symptoms can also be present in the general pediatric population. In the CLoCk study, no difference was found in the distribution of mental health scores and well-being between those who tested positive and negative for coronavirus. Regarding their health-related quality of life, children who previously had COVID-19 were more likely to report problems with mobility, doing usual activities, pain or discomfort, and were more likely to be worried or sad than those who had not.21 Further controlled studies are needed to separate the effect of the pandemic era from the infection itself.
As there is no universal evidence-based treatment available for children with LCS, we could only offer non-specific supportive therapy, as part of individualized complex rehabilitation based on NICE guidance.7 In some cases, we were able to provide specific therapeutic approaches, like smell training for dys- or anosmia, L-thyroxin for hypothyroidism, inhaled corticosteroids for obstructive diseases.
According to the literature, the acute illness remains hidden or invalidated by laboratory tests in a significant proportion of children, therefore proving a previous SARS-CoV-2 infection can be challenging.5 In contrast, we observed that 90% of children were proven with a laboratory test to have had a previous illness by the time they visited our clinic. It is noteworthy that the majority of the LCS children had a mild acute COVID sequel, similar to Ashkenazi et al.’s findings.8
Our sample displays an almost 2:1 female-to-male ratio, with females having more LC symptoms. Stephenson et al. and Behnood et al. reported females, older children, and those with mental or physical comorbidities as risk factors for having more symptoms.19,21 Most of the children presented in our clinic were school-aged, consistent with recent British,27 and Israeli reports.8 It is questionable, however, whether younger children also experienced lingering symptoms, being less able to verbalize them efficiently.
Our study lacks a control group, which makes our results non-comparable to an average pediatric population regarding symptoms, quality of life, laboratory results, and other findings, therefore, our goals cannot exceed the detailed description of pediatric LCS. We should also note that families’ perceptions and recollections of the pre-pandemic condition can bias the answers given to our survey. Also, the described duration of symptoms could be influenced by the opening date of our outpatient clinic as well as our limited capacity. The appointment registration process was executed online primarily, and this may have restricted the opportunity for some families. However, supported by the systematic data recording and in person multi-disciplinary assessment we could provide valuable clinical data for future investigations and clinicians examining children with LCS.
In conclusion, we could diagnose clinically important conditions with therapeutic consequences, but the percentage of those findings was low. The most probable, strongly COVID-19-related conditions were the frequently observed olfactory and taste disturbances. The observed concerningly high proportion of mental health problems adds to the burden of the disease. We had patients ranging from those who essentially reported no disturbances in their quality of life, to others having 33 different complaints at a time, with the inability to even dress. It was therefore utterly complicated to reflect the true variability of described complaints and clinical picture in numbers without predefined guidance. Our impression is that setting up complaint-centered outcome measures might be a useful option for better interpreting the severity of the disease. It would also be highly important to establish evidence-based pediatric guidelines, potentially allowing a cost optimization by narrowing down the recommended list of examinations. Our results, however, seem to support that some formerly suggested examinations such as thyroid screening28 be retained in future guidelines, as we found (occasionally asymptomatic) autoimmune thyroiditis in 7% of all cases. Until larger, controlled prospective analytical studies are conducted in the pediatric population, it remains ambiguous to rationalize the essential parts of basic checkups, which currently sets a heavy burden on both the healthcare system and patients. Currently, there is no specific treatment option for LCS. A case-control study showed a significantly reduced frequency of LCS in fully vaccinated adults compared to unvaccinated controls.29 Therefore, trials approving vaccination in children starting from 5 years of age might open new opportunities for the prevention of LCS in children.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Irfan, O. et al. Clinical characteristics, treatment and outcomes of paediatric Covid-19: a systematic review and meta-analysis. Arch. Dis. Child. 106, 440–448 (2021).
Ludvigsson, J. F. Systematic review of Covid‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 109, 1088–1095 (2020).
Ludvigsson, J. F. Reporting suspicions of long Covid in children is justified during this global emergency. Acta Paediatrica 110, 1373–1373 (2021).
Soriano, J. B., Murthy, S., Marshall, J. C., Relan, P. & Diaz, J. V. A clinical case definition of post-Covid-19 condition by a Delphi consensus. Lancet Infect. Dis. 22, e102–e107 (2021).
Sah, P. et al. Asymptomatic SARS-Cov-2 infection: a systematic review and meta-analysis. Proc. Natl Acad. Sci. USA 118, e2109229118 (2021).
Nalbandian, A. et al. Post-acute Covid-19 syndrome. Nat. Med. 27, 601–615 (2021).
Venkatesan, P. NICE guideline on long Covid. Lancet Respir. Med. 9, 129 (2021).
Ashkenazi-Hoffnung, L. et al. Long Covid in children: observations from a designated pediatric clinic. Pediatr. Infect. Dis. J. 40, e509–e511 (2021).
Osmanov, I. M. et al. Risk factors for long Covid in previously hospitalised children using the Isaric Global Follow-up Protocol: a prospective cohort study. Eur. Respir. J. 59, 2101341 (2021).
Morand, A. et al. Similar patterns of [(18)F]-FDG brain pet hypometabolism in paediatric and adult patients with long Covid: a paediatric case series. Eur. J. Nucl. Med. Mol. Imaging 49, 913–920 (2022).
Erol, N., Alpinar, A., Erol, C., Sari, E. & Alkan, K. Intriguing new faces of Covid-19: persisting clinical symptoms and cardiac effects in children. Cardiol. Young 32, 1085–1091 (2022).
Buonsenso, D. et al. Evidence of lung perfusion defects and ongoing inflammation in an adolescent with post-acute sequelae of SARS-Cov-2 infection. Lancet Child Adolesc. Health 5, 677–680 (2021).
Panda, P. K. et al. Psychological and behavioral impact of lockdown and quarantine measures for Covid-19 pandemic on children, adolescents and caregivers: a systematic review and meta-analysis. J. Trop. Pediatr. 67, fmaa122 (2021).
Mansourian, M., Ghandi, Y., Habibi, D. & Mehrabi, S. Covid-19 infection in children: a systematic review and meta-analysis of clinical features and laboratory findings. Arch. Pediatr. 28, 242–248 (2021).
Shah, W., Hillman, T., Playford, E. D. & Hishmeh, L. Managing the long term effects of Covid-19: summary of NICE, SIGN, and RCGP Rapid Guideline. BMJ 372, n136 (2021).
Joubert, K. et al. Results of the Hungarian Longitudinal Child Growth Study: From Birth to the Age of 18 Years (I.). Report No. 83. 64–65 (Hungarian Demographic Research Institute, 2006).
Epskamp, S., Cramer, A. O. J., Waldorp, L. J., Schmittmann, V. D. & Borsboom, D. Qgraph: network visualizations of relationships in psychometric data. J. Stat. Softw. 48, 1–18 (2012).
Malik, P. et al. Post-acute Covid-19 syndrome (PCS) and health-related quality of life (HRQOL)-a systematic review and meta-analysis. J. Med. Virol. 94, 253–262 (2021).
Behnood, S. A. et al. Persistent symptoms following SARS-Cov-2 infection amongst children and young people: a meta-analysis of controlled and uncontrolled studies. J. Infect. 84, 158–170 (2022).
da Silva, F. C. T. & Neto, M. L. R. Psychological effects caused by the Covid-19 pandemic in health professionals: a systematic review with meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 104, 110062 (2021).
Stephenson, T. et al. Physical and mental health 3 months after SARS-Cov-2 infection (long Covid) among adolescents in England (Clock): a national matched cohort study. Lancet Child Adolesc. Health 6, 230–239 (2022).
Joshee, S., Vatti, N. & Chang, C. Long-term effects of Covid-19. Mayo Clin. Proc. 97, 579–599 (2022).
Rocha, K. O., Zanuncio, V. V., Freitas, B. A. C. & Lima, L. M. “Covid toes”: a meta-analysis of case and observational studies on clinical, histopathological, and laboratory findings. Pediatr. Dermatol. 38, 1143–1149 (2021).
Feghali, K., Atallah, J. & Norman, C. Manifestations of thyroid disease post Covid-19 illness: report of hashimoto thyroiditis, graves’ disease, and subacute thyroiditis. J. Clin. Transl. Endocrinol. Case Rep. 22, 100094 (2021).
Murugan, A. K. & Alzahrani, A. S. SARS-Cov-2: emerging role in the pathogenesis of various thyroid diseases. J. Inflamm. Res. 14, 6191–6221 (2021).
Brodin, P. Immune determinants of Covid-19 disease presentation and severity. Nat. Med. 27, 28–33 (2021).
Molteni, E. et al. Illness duration and symptom profile in symptomatic Uk school-aged children tested for SARS-Cov-2. Lancet Child Adolesc. Health 5, 708–718 (2021).
Lui, D. T. W. et al. Insights from a prospective follow-up of thyroid function and autoimmunity among Covid-19 survivors. Endocrinol. Metab. 36, 582–589 (2021).
Antonelli, M. et al. Risk factors and disease profile of post-vaccination SARS-Cov-2 infection in UK users of the Covid symptom study app: a prospective, community-based, nested, case-control study. Lancet Infect. Dis. 22, 43–55 (2021).
Acknowledgements
We would like to acknowledge the help of our colleagues Orsolya Máthé, Erika Kiss, Réka Csizmadia-Kiss, Dr. Anna Végh, Dr. Lilla Szeifert, Dr. Andrea Tölgyesi, Dr. Lajos Kovács, Dr. Dóra Krikovszky, Dr. Nóra Béres, Dr. Áron Cseh, Dr. Ildikó Várkonyi, Dr. Anna Nyitrai, Dr. Tímea Seszták, Dr. Illés Balogh, Dr. Helga Kraxner, Dr. Viktor Farkas, Dr. Kristóf Farkas, Dr. Tamás Constantin, Dr. Marcell Imrei, Edit Ubora, Katalin Ernszt, and Hajnalka Négrádi and our colleagues from the Emergency Unit and Robert James Robson.
Funding
Open access funding provided by Semmelweis University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception. R.G. conceptualized and designed the study, collected data, managed initial data curation and wrote the manuscript, reviewed and revised the final manuscript. P.K. conceptualized and designed the study, supervised the investigation, wrote the manuscript, reviewed and revised the final manuscript. V.H. conceptualized and designed the study, collected data, managed initial data curation, and wrote the manuscript. F.K. conceptualized and designed the study, collected data, managed initial data curation, and wrote the manuscript. B.T. conceptualized and designed the study, analyzed and interpreted the results, and wrote the manuscript. J.K. helped to conceptualize and design the study, collected data, managed initial data curation, and helped draft the initial manuscript. A.M. participated in designing the study, collected data, supported project administration, and helped draft the initial manuscript. E.Z. participated in designing the study, collected data, supported project administration, and helped draft the initial manuscript. J.T. helped to conceptualize and design the study, conducted the initial analyses, analyzed and interpreted the results, contributed to data visualization, and helped draft the initial manuscript. D.S.V. helped to conceptualize and design the study, analyzed and interpreted the results, supervised data analysis, contributed to data visualization, and critically reviewed the manuscript for important intellectual content. A.J.S. conceptualized and designed the study, supervised the investigation, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
No consent was required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
OpenAccess This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Garai, R., Krivácsy, P., Herczeg, V. et al. Clinical assessment of children with long COVID syndrome. Pediatr Res 93, 1616–1625 (2023). https://doi.org/10.1038/s41390-022-02378-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02378-0
This article is cited by
-
What are the key pediatric public policy priorities as the COVID-19 pandemic persists?
Pediatric Research (2023)
-
Thyroid disturbances after COVID-19 and the effect of vaccination in children: a prospective tri-center registry analysis
European Journal of Pediatrics (2023)