Abstract
Background
Low levels of insulin-like growth factor-1 (IGF-1) protein in preterm human infants are associated with bronchopulmonary dysplasia (BPD). We used our preterm lamb model of BPD to determine (1) dosage of recombinant human (rh) IGF-1 bound to binding protein-3 (IGFBP-3) to reach infant physiologic plasma levels; and (2) whether repletion of plasma IGF-1 improves pulmonary and cardiovascular outcomes.
Methods
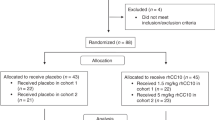
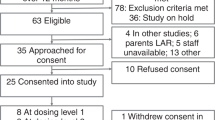
Group 1: normal, unventilated lambs from 128 days gestation through postnatal age 5 months defined normal plasma levels of IGF-1. Group 2: continuous infusion of rhIGF-1/rhIGFBP-3 (0.5, 1.5, or 4.5 mg/kg/day; n = 2) for 3 days in mechanically ventilated (MV) preterm lambs determined that 1.5 mg/kg/day dosage attained physiologic plasma IGF-1 concentration of ~125 ng/mL, which was infused in four more MV preterm lambs.
Results
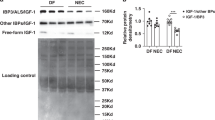
Group 1: plasma IGF-1 protein increased from ~75 ng/mL at 128 days gestation to ~220 ng/L at 5 months. Group 2: pilot study of the optimal dosage (1.5 mg/kg/day rhIGF-1/rhIGFBP-3) in six MV preterm lambs significantly improved some pulmonary and cardiovascular outcomes (p < 0.1) compared to six MV preterm controls. RhIGF-1/rhIGFBP-3 was not toxic to the liver, kidneys, or lungs.
Conclusions
Three days of continuous iv infusion of rhIGF-1/rhIGFBP-3 at 1.5 mg/kg/day improved some pulmonary and cardiovascular outcomes without toxicity.
Impact
-
Preterm birth is associated with rapid decreases in serum or plasma IGF-1 protein level. This decline adversely impacts the growth and development of the lung and cardiovascular system. For this pilot study, continuous infusion of optimal dosage of rhIGF-1/rhIGFBP-3 (1.5 mg/kg/day) to maintain physiologic plasma IGF-1 level of ~125 ng/mL during mechanical ventilation for 3 days statistically improved some structural and biochemical outcomes related to the alveolar formation that would favor improved gas exchange compared to vehicle-control. We conclude that 3 days of continuous iv infusion of rhIGF-1/rhIGFBP-3 improved some physiological, morphological, and biochemical outcomes, without toxicity, in mechanically ventilated preterm lambs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Northway, W. H. Jr., Rosan, R. C. & Porter, D. Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N. Eng. J. Med. 276, 357–368 (1967).
Stoll, B. J. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 314, 1039–1051 (2015).
Voller, S. B. et al. Cord blood biomarkers of vascular endothelial growth (VEGF and sFlt-1) and postnatal growth: a preterm birth cohort study. Early Hum. Dev. 90, 195–200 (2014).
Olmos, A. et al. Associations between insulin-like growth factor I, vascular endothelial growth factor and its soluble receptor 1 in umbilical serum and endothelial cells obtained from normotensive and preeclamptic pregnancies. Growth Factors 31, 123–129 (2013).
Hellström, A. et al. Insulin-like growth factor 1 has multisystem effects on foetal and preterm infant development. Acta Paediatr. 105, 576–586 (2016).
De Paepe, M. E. et al. Growth of pulmonary microvasculature in ventilated preterm infants. Am. J. Respir. Crit. Care Med. 173, 204–211 (2006).
Lassarre, C. et al. Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr. Res. 29, 219–225 (1991).
Li, J. et al. The IGF-I/IGF-R1 pathway regulates postnatal lung growth and is a nonspecific regulator of alveologenesis in the neonatal rat. Am. J. Physiol. Lung Cell Mol. Physiol. 304, L626–L637 (2013).
Ley, D. et al. rhIGF-1/rhIGFBP-3 in preterm infants: a phase 2 randomized controlled trial. J. Pediatr. 206, 56–65.e58 (2019).
Seedorf G., et al. rhIGF-1/BP3 preserves lung growth and prevents pulmonary hypertension in experimental BPD. Am. J. Respir. Crit. Care Med. 201, 1120–1134 (2020).
Albertine, K. H. et al. Chronic lung injury in preterm lambs: disordered respiratory tract development. Am. J. Respir. Crit. Care Med. 159, 945–958 (1999).
Reyburn, B. et al. Nasal ventilation alters mesenchymal cell turnover and improves alveolarization in preterm lambs. Am. J. Respir. Crit. Care Med. 178, 407–418 (2008).
Albertine, K. H. et al. Chronic lung disease in preterm lambs: effect of daily vitamin A treatment on alveolarization. Am. J. Physiol. Lung Cell Mol. Physiol. 299, L59–L72 (2010).
Rehan, V. K. et al. Mechanism of reduced lung injury by high-frequency nasal ventilation in a preterm lamb model of neonatal chronic lung disease. Pediatr. Res. 70, 462–466 (2011).
Joss-Moore L. A., et al. Alveolar formation is dysregulated by restricted nutrition but not excess sedation in preterm lambs managed by non-invasive support. Pediatr. Res. 80, 719–728 (2016).
Dahl, M. J. et al. Former-preterm lambs have persistent alveolar simplification at 2 and 5 months corrected postnatal age. Am. J. Physiol. Lung Cell Mol. Physiol. 315, L816–L833 (2018).
Liu, M. et al. Transforming growth factor-induced protein promotes NF-kappaB-mediated angiogenesis during postnatal lung development. Am. J. Respir. Cell Mol. Biol. 64, 318–330 (2021).
Dahl, M. J. et al. Early extubation to noninvasive respiratory support of former preterm lambs improves long-term respiratory outcomes. Am. J. Physiol. Lung Cell Mol. Physiol. 321, L248–L262 (2021).
Null, D. M. et al. High-frequency nasal ventilation for 21 d maintains gas exchange with lower respiratory pressures and promotes alveolarization in preterm lambs. Pediatr. Res. 75, 507–516 (2014).
Hsia, C. C., Hyde, D. M., Ochs, M. & Weibel, E. R. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am. J. Respir. Crit. Care Med. 181, 394–418 (2010).
Unsworth, W. P., Taylor, J. A. & Robinson, J. E. Prenatal programming of reproductive neuroendocrine function: the effect of prenatal androgens on the development of estrogen positive feedback and ovarian cycles in the ewe. Biol. Reprod. 72, 619–627 (2005).
Löfqvist, C. et al. Low postnatal serum IGF-I levels are associated with bronchopulmonary dysplasia (BPD). Acta Paediatr. 101, 1211–1216 (2012).
Chung, J. K. et al. Development and verification of a pharmacokinetic model to optimize physiologic replacement of rhIGF-1/rhIGFBP-3 in preterm infants. Pediatr. Res. 81, 504–510 (2017).
Jakkula, M. et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am. J. Physiol. Lung Cell Mol. Physiol. 279, L600–L607 (2000).
Le Cras, T. D. et al. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am. J. Physiol. Lung Cell Mol. Physiol. 283, L555–L562. (2002).
Hellström, A. et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc. Natl Acad. Sci. USA 98, 5804–5808 (2001).
Epaud, R. et al. Knockout of insulin-like growth factor-1 receptor impairs distal lung morphogenesis. PLoS One 7, e48071 (2012).
Bach, L. A. Endothelial cells and the IGF system. J. Mol. Endocrinol. 54, R1–R13 (2015).
Jacobo, S. M. & Kazlauskas, A. Insulin-like growth factor 1 (IGF-1) stabilizes nascent blood vessels. J. Biol. Chem. 290, 6349–6360 (2015).
Zhang, M. et al. Insulin-like growth factor 1/insulin-like growth factor 1 receptor signaling protects against cell apoptosis through the PI3K/AKT pathway in glioblastoma cells. Exp. Ther. Med. 16, 1477–1482 (2018).
Liu, Q. et al. Insulin-like growth factor 1 receptor-mediated cell survival in hypoxia depends on the promotion of autophagy via suppression of the PI3K/Akt/mTOR signaling pathway. Mol. Med. Rep. 15, 2136–2142 (2017).
Remacle-Bonnet, M. et al. Membrane rafts segregate pro- from anti-apoptotic insulin-like growth factor-I receptor signaling in colon carcinoma cells stimulated by members of the tumor necrosis factor superfamily. Am. J. Pathol. 167, 761–773 (2005).
Kooijman, R. Regulation of apoptosis by insulin-like growth factor (IGF)-I. Cytokine Growth Factor Rev. 17, 305–323 (2006).
Kimura, T. et al. Disposition of recombinant human insulin-like growth factor-I in normal and hypophysectomized rats. Biol. Pharm. Bull. 17, 310–315 (1994).
Mizuno, N. et al. Kinetic analysis of the disposition of insulin-like growth factor 1 in healthy volunteers. Pharm. Res. 18, 1203–1209 (2001).
FDA. Increlex Insert. Reference ID: 3517143. https://www.accessdata.fda.gov 7–8 (2014).
Acknowledgements
We thank the dozens of undergraduate students and medical students who learned and provided neonatal intensive care to the preterm lambs. We also thank Angela Presson, PhD, MS, at the University of Utah for guidance for the study’s pilot design and statistical analysis, as well as Jennifer Bosco, PhD, and Bettina Stack-Logue, PhD, at Takeda Pharmaceutical Company who provided in vitro pharmacology support to characterize the effect of rhIGF-1 on sheep IGF-1 receptors.
Funding
Supported by research grant awards from Takeda (formerly Shire) Pharmaceuticals and NIH R01 HL110002 (K.H.A.) and T35 HL007744 (supported C.M.), and the Division of Neonatology, Department of Pediatrics, University of Utah.
Author information
Authors and Affiliations
Contributions
K.H.A.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. M.J.D.: Substantial contributions to acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. A.R.: Substantial contributions to acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. E.D.: Substantial contributions to acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. A.N.: Substantial contributions to acquisition of data, or analysis and interpretation of data; and final approval of the version to be published. S.B.: Substantial contributions to acquisition of data, or analysis and interpretation of data; and final approval of the version to be published. C.M.: Substantial contributions to acquisition of data, or analysis and interpretation of data; and final approval of the version to be published. Z.W.: Substantial contributions to acquisition of data, or analysis and interpretation of data; and final approval of the version to be published. H.Y.: Substantial contributions to acquisition of data, or analysis and interpretation of data; and final approval of the version to be published. B.Y.: Substantial contributions to acquisition of data, or analysis and interpretation of data; and final approval of the version to be published. D.M.N.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. D.K.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. J.-K.C.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. Z.Z: Substantial contributions to acquisition of data, or analysis and interpretation of data; and final approval of the version to be published. N.B.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. G.C.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. R.W.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
A competing interest is identified because the study was funded by an independent research grant award from Takeda Pharmaceuticals to the University of Utah for this study to be done in Dr Albertine’s lamb intensive care unit.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Albertine, K.H., Dahl, M.J., Rebentisch, A. et al. Pilot dose-ranging of rhIGF-1/rhIGFBP-3 in a preterm lamb model of evolving bronchopulmonary dysplasia. Pediatr Res 93, 1528–1538 (2023). https://doi.org/10.1038/s41390-022-02272-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02272-9