Abstract
Background
Obstructive sleep apnea in infants with Pierre Robin sequence is sleep-position dependent. The influence of sleep position on obstructive events is not established in other infants.
Methods
We re-evaluated ten-year pediatric sleep center data in infants aged less than six months, with polysomnography performed in different sleep positions. We excluded infants with syndromes, genetic defects, or structural anomalies.
Results
Comparison of breathing between supine and side sleeping positions was performed for 72 infants at the median corrected age of 4 weeks (interquartile range (IQR) 2-8 weeks). Of the infants, 74% were male, 35% were born prematurely, and 35% underwent study because of a life-threatening event or for being a SIDS sibling. Upper airway obstruction was more frequent (obstructive apnea-hypopnea index (OAHI), p < 0.001), 95th-percentile end-tidal carbon dioxide levels were higher (p = 0.004), and the work of breathing was heavier (p = 0.002) in the supine than in the side position. Median OAHI in the supine position was 8 h−1 (IQR 4–20 h−1), and in the side position was 4 h−1 (IQR 0-10 h−1).
Conclusions
Obstructive upper airway events in young infants are more frequent when supine than when sleeping on the side.
Impact
-
The effect of sleep position on obstructive sleep apnea is not well established in infants other than in those with Pierre Robin sequence.
-
A tendency for upper airway obstruction is position dependent in most infants aged less than 6 months. Upper airway obstruction is more common, end-tidal carbon dioxide 95th-percentile values higher, and breathing more laborious in the supine than in the side-sleeping position.
-
Upper airway obstruction and obstructive events have high REM sleep predominance.
-
As part of obstructive sleep apnea treatment in young infants, side-sleeping positioning may prove useful.
Similar content being viewed by others
Introduction
Infants commonly experience single obstructive events during sleep.1,2,3 However, severe adult-type obstructive sleep apnea (OSA) is rare and often related to syndromes such as Pierre Robin sequence (PRS).1,3,4 Obstructive events in infants show REM-sleep dominancy, and they do not commonly interrupt sleep to the same degree as in adults.1 Gastroesophageal reflux appears to lead to increased numbers of obstructive events, although the reflux and apnea episodes do not generally appear simultaneously.5 The significance of OSA in infants is not yet established. Obstructive events have been associated with increased risk for sudden infant death syndrome (SIDS).6,7 Currently, no consensus exists: neither as to criteria for degree of upper airway obstruction should be considered OSA nor when OSA should be treated.1
OSA is sleep position dependent in adults, older children and in infants with PRS.4,8,9,10 Only one polysomnography (PSG) -based study concerns the effect of sleep position on upper airway obstruction in infants without any syndrome. Orr and associates11 showed in 65 infants that upper airway obstruction was neither position- nor sleep-stage dependent. However, in their study the detection of airflow detection was not based on detection of reliable flow signal, but on carbon-dioxide signal.
After recognition of position dependency of OSA in infants with PRS4 and preliminary observations in other infants, we included position control in our infant PSG protocol and, whenever upper airway obstruction was suspected or observed, we performed PSG in supine and side-sleep positions. The aim of this study was to determine the effects of sleep position on upper airway obstruction in infants less than six months of age.
Methods
Study design and patients
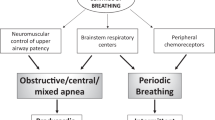
We evaluated our pediatric sleep unit data from 2011 to 2021, including infants less than six months old showing obstructive events but without predominance of central breathing disorders and periodic breathing in PSG (Fig. 1). We excluded infants with recognized facial and upper airway abnormalities, syndrome, or genetic abnormalities. The Helsinki University ethics committee (HUS/3186/2019) and New Children’s Hospital Institutional Review Board (Project #4980) approved the study protocol.
ALTE apparent life-threatening event, ECG electrocardiogram, EEG electroencephalogram, EMG electromyogram, EOG electro-oculogram, EtCO2 end-tidal carbon dioxide, IQR interquartile range, MoveHF movement sensor high-frequency band representing general movements, MoveLF movement sensor low-frequency signal representing respiratory movements, Pleth oximeter plethysmography, Rib respiratory induction plethysmography, SIDS sudden infant death syndrome, SpO2 pulse oximeter oxyhemoglobin saturation, TcCO2 transcutaneous carbon dioxide.
We performed sleep studies in supine and side positions if obstructive events were suspected or observed during online PSG analysis. Infants were also placed in a prone position on a few occasions. At least one cycle of rapid-eye-movement sleep (REM) and of non-REM sleep was recorded in each position. The sleep studies took place during daytime in infants less than three months old, and during night-time in older infants.
Polysomnography
The PSG setup and analysis followed the recommendations of the American Academy of Sleep Medicine (AASM).12 We used Embla N700 (Natus Medical Inc., WI) prior to 2018, and the SOMNOTM HD (SOMNOmedics GmbH, Germany) PSG systems thereafter. The PSG protocol (Fig. 1) comprised monitoring of four electroencephalogram channels (Cz-Fz, Cz-O2, C4-M1, O2-M1), two electro-oculography channels, chin- and diaphragm electromyography (EMG), nasal airflow by pressure transducer, two respiratory inductive plethysmography belts, electrocardiography, two pulse oximeters (SpO2) with a two- to-four second averaging interval (Embla or SOMNOTM HD, and Masimo Radical Pulse CO-Oximeter, Masimo Co, CA), end-tidal carbon dioxide (EtCO2) (CAP10 Capnograph, Medlab medizinische Diagnosegeräte GmbH, Germany), transcutaneous carbon dioxide (TcCO2) (SenTec Inc., MO), a movement sensor mattress (Emfit Ltd, Finland), a position sensor, and a synchronized video recording. Thermistor was added to the setup if oral breathing was suspected or noted.
All PSG recordings we reanalyzed (T.K.) blinded to position, and we visually scored the sleep stage and respiratory events. Central, obstructive, and mixed apneas, and obstructive hypopneas were recognizable based on airflow, diaphragm EMG activity, and respiratory movement. Work of breathing (WOB) we estimated from the diaphragm EMG activity and graded from 0 to 2, where 0 represented normal, 1 slightly increased inspiratory EMG activity, and 2 laborious breathing with clearly increased activity (Supplementary Fig. S1). We performed WOB scoring twice in a blinded manner with an over six-month time interval between scoring sessions to calculate inter-scorer (T.K.) repeatability (Supplementary Table S1). Oxygen desaturations over 3% (ODI≥3) were analyzed both separately and in relation to the preceding apnea or hypopnea (ODI≥3 central apnea / obstructive apnea/obstructive hypopnea/mixed apnea). We used self-developed (T.K.) special purpose software to complete detailed SpO2, TcCO2 analyses, and breath-by-breath analyses of EtCO2 and of breathing frequency. Software allowed us to compare combined-event data such as over 3% desaturation events (OD≥3) in relation to obstructive, mixed, and central apneas and obstructive hypopneas.
Statistical methods and analysis
The statistical analysis required IBM® SPSS® Statistics software version 25 and OriginPro 2022. Because PSG parameters were not normally distributed, we selected non-parametric tests for the analysis. The Wilcoxon signed-rank test served for the pairwise comparison between sleep positions and sleep stages. We used Mann-Whitney Test to compare the changes in breathing parameter results between supine and side sleeping positions in the two groups of infants with different order of sleeping position. Kappa statistics served to calculate repeatability of WOB scoring. The level of significance we set at p < 0.05.
Results
During the ten-year study period, a total of 786 PSG studies took place with infants less than six months of age. After the application of our exclusion criteria, a total of 92 infants presented with obstructive events, and 72 were eligible for comparison between supine and side-sleeping positions. Because these positions were preferable sleeping positions, were therefore placed only 15 infants in a prone position (See Supplementary Fig. S2). The median corrected age of 72 infants was 4 weeks (IQR 2-8 weeks), with 53 (74%) male, 25 (35%) were born prematurely, and another 25 (35%) were studied because of an earlier apparently life-threatening event (ALTE) or because each was the sibling of an infant who had died from SIDS (Fig. 1). Findings for these two subgroups: the premature infants and the ALTE infants or SIDS-siblings, were similar to those for the rest of infants.
Median PSG recording time was 4.0 h (IQR 3.2–4.7 h) and total sleep time 2.4 h (IQR 2.0–2.9 h). Out of the 72 infants who slept both in supine and in side positions, 55 (76%) were placed first in the supine position. The groups with different sleep position order had similar changes in number of apneas, hypopneas and oxygen desaturations events (p = 0.16 – 0.68).
Most of these infants experienced with few obstructive events during our sleep study, but all experienced obstructive events at the rate of at least one per hour of sleep when supine (Fig. 2). Of the infants, 58 (63%) had an obstructive apnea-hypopnea index (OAHI) over 5 per hour.
Number of infants according to obstructive apnea-hypopnea index (OAHI). Red vertical lines indicate OAHI levels of 1 (zoomed figures) and 5 per hour during sleep. OAHI shifts from higher values towards lower, normal values from supine- to side-sleeping position. In supine, all infants (72) had OAHI above 1 per hour and 48 (67%) had above 5 per hour of sleep. In the side-sleeping position, 51 (71%) infants had OAHI above 1 per hour and 30 (42%) had above 5 per hour during sleep.
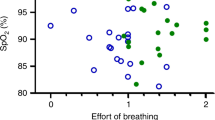
The appearance of upper airway obstruction was both sleep-position and sleep-stage dependent (Table 1, Figs. 2–4). OAHI was higher in the supine than on the side position (p < 0.001) and in REM than in non-REM sleep (p < 0.0001). Estimated WOB was more laborious in 13 (18%) infants (p = 0.01), but EtCO2 95th-percentile values were systematically higher (p = 0.004) on the supine than on the side position. Only one infant (1%) experienced with heavier WOB in the side than in the supine position. Most of the oxygen desaturations observed in these infants were related to central apneas, and sleep position did not affect their appearance (Table 1).
Side sleeping position reduced the number of obstructive events, and of end-tidal carbon dioxide 95th percentile levels, and in 13 (18%) infants, reduced work of breathing when compared to values in a supine-sleeping position. EtCO2P95 95th-percentile level of end-tidal carbon dioxide, OAHI obstructive apnea-hypopnea index, ODI≥3OAHI oxyhemoglobin desaturations index of ≥3% from baseline following obstructive and mixed apneas, or obstructive hypopneas, SpO2 MinOAHI minimum pulse oximeter hemoglobin saturation value following obstructive and mixed apneas, or obstructive hypopneas, TcCO2P95 95th-percentile level of transcutaneous carbon dioxide.
Recording summary of a two-month-old infant with position dependent obstructive sleep apnea. The infant first slept supine followed by recording in a side position. Obstructive and mixed apneas occurred almost entirely in the supine-sleeping position during REM sleep. In the second horizontal panel, one vertical bar presents one event. Brown vertical shadow bars cover periods of wakefulness with unreliable signal recording. BP blood pressure. Values calculated from pulse transit time. EtCO2 end-tidal carbon dioxide, Respfreq breathing frequency, SpO2 Desat pulse oximeter oxyhemoglobin desaturation ≥3%, TcCO2 transcutaneous carbon dioxide.
Discussion
We have shown in 72 infants aged less than six months that the appearance of upper airway obstruction is consistently both sleep-position and sleep-stage dependent. Obstructive events were more common, end-tidal carbon dioxide 95th-percentile levels higher, and breathing was more laborious in the supine than in the side position. As well established, obstructive events here were also much more common in REM than in non-REM sleep.
OSA and sleep position
In children and adults, despite the fact that upper airway narrowing and increased tendency for upper airway collapse have an underlying structural cause, OSA is often sleep-position dependent. OSA is most often more severe in the supine than in the side- or prone-sleeping positions.8,9 The same position dependency it true for young infants with PRS and small chin,4,10 but also, as shown in our study, is true for most infants with OSA even without any clear predisposing structural deficit. The anatomical sites of upper airway obstruction in young infants are similar to sites in adults with OSA.13
OSA during the first three months seems to have its own characteristics, and as in our study cohort, it may appear without any noticeable anatomical abnormality.13 OSA in young infants is evident especially in young infants with gastroesophageal reflux,5,14,15 in premature infants,16,17,18,19 and in infants with earlier ALTE.7,20 Regardless of the underlying condition, infant OSA seems to be resolved by the age of four to eight months.4,21,22,23
In a study by Pereira et al.24, 50 infants older than ours, aged 8 to 12 months with mild OSA, showed no significant effect of sleep position on OSA. In children with normal facial structure who were older than six months, OSA has been most closely related to adenoidal or tonsillar hypertrophy.17,25 This difference in OSA etiology is also likely to explain the differing findings between younger and older infants. In our study group, clinical and PSG follow-up showed the resolution of obstructive sleep apnea in most of our infant before they reached 4–8 months old.
Most of our infants had mild OSA if compared, for example, to the OSA observed in many PRS infants.4 All of ours had a normal baseline SpO2 level, and the great majority also had normal EtCO2 and TcCO2 levels, together with a low number of oxygen desaturations in related to obstructive and mixed apneas and obstructive hypopneas. Approximately half of our infants displayed normal work of breathing while in the supine-, and two-thirds while in the side-sleeping position. The reduction in 95th-percentile levels of EtCO2 from supine- to side position may reflect a resolution of partial upper airway obstruction, but no cause for this reduction could be unequivocally determined.
The effect of sleep positioning observed here may not be considered sufficient to treat OSA in some infants. However, side-position for sleep may still prove useful as accessory to other forms of treatment such as high-flow nasal cannula (HFNC) or continuous positive airway pressure (CPAP).
Role of sleep positioning in infants
Currently, the supine sleep position is recommended for all infants as part of SIDS-reduction campaigns.26 No exceptions attract attention, despite infants such as those with PRS seeming to benefit from the side-sleep positioning.4 One estimated odds ratio (OR) for SIDS between the supine and prone sleeping position is 4.46, and is 1.36 between supine and side sleeping positions.27 How real is the risk of SIDS being related to side-sleep positioning if side or prone positioning were applied as a part of OSA treatment? Prior to SIDS sleeping campaigns in the 1970’s and 1980’s, SIDS rates in Finland ranged from 0.5 to 0.6 per 1000 live births. In order to establish the safety of any type of OSA treatment, the study group should exceed 7 600 infants when comparing treatment safety to sleep position treatment with prone sleep positioning, and over 76,000 for comparing the treatment to side-sleep positioning. This simple power calculation demonstrates the low risk level related to side sleep positioning. It may not be valid to apply the standard sleep position recommendation to a specific infant population from whom side-sleep positioning may be otherwise beneficial.4
Study limitations
Here, most of our PSG studies were done as daytime recording for clinical purposes. The recordings were approximately half of the length as obtainable by standard overnight PSG. The recordings were continued in each sleeping position as long as needed for appropriate clinical decision-making, each recording at least one full sleep cycle including non-REM and REM periods in each position. Daytime recordings have their pros and cons, but generally the literature supports their clinical use in infants age less than three months.3,28,29 In this age group, infants generally sleep enough during daytime for reliable PSG analysis.30,31 In Finland, daytime recordings are more readily available than whole-night recordings. Daytime recordings allowed a pediatric pulmonologist’s online surveillance of PSG if indicated. Thus, for example our study group, in the majority of cases, PSG studies were continued until proper clinical decision-making was possible in respect to sleep position. Despite the time limitations, we do not conclude that whole-night PSG recording would have significantly affected the study conclusions.
We used diaphragm EMG for a rough estimate of breathing effort. EMG was recorded by surface electrodes, and measurement amplitude was compared to baseline levels during quiet breathing, and to baseline background activity. Such a measurement is difficult to calibrate properly and is affected by signal filtration and, impedance, and by sleep stage (Supplementary Fig. S1).32 We did not have automatic breath-by-breath analysis of diaphragm EMG similar to what we had for EtCO2 and breathing frequency analysis. A proper estimation of breathing effort would have required esophageal pressure measurement,13,33 and diaphragm and esophageal pressure measurement are expected to show only modest correlation.34 Despite these limitations, we find diaphragm EMG useful, because no readily available alternatives exist.4 Our WOB scoring showed good repeatability (Supplementary Table S1), and we believe the scoring reflects the real WOB changes between sleep positions with reasonable accuracy.
Conclusions
In most infants without any noticeable structural facial or airway anomaly or syndrome, the upper airway obstruction was both sleep-position and sleep-stage dependent, with the side-sleep position reducing the frequency of obstructive events when compared with the supine position.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bandyopadhyay, A. & Daftary, A. S. Obstructive sleep apnea in infants during the first year of life: what the pediatrician needs to know. Clin. Pediatrics 59, 752–759 (2020).
Brockmann, P. E., Poets, A. & Poets, C. F. Reference values for respiratory events in overnight polygraphy from infants aged 1 and 3 months. Sleep. Med. 14, 1323–1327 (2013).
Daftary, A. S., Jalou, H. E., Shively, L., Slaven, J. E. & Davis, S. D. Polysomnography reference values in healthy newborns. J. Clin. Sleep. Med. 15, 437–443 (2019).
Kukkola, H. K., Vuola, P., Seppä-Moilanen, M., Salminen, P. & Kirjavainen, T. Pierre Robin sequence causes position-dependent obstructive sleep apnoea in infants. Arch. Dis. Child 106, 954–960 (2021).
Arad-Cohen, N., Cohen, A. & Tirosh, E. The relationship between gastroesophageal reflux and apnea in infants. J. Pediatrics 137, 321–326 (2000).
Kahn, A. et al. Lack of temporal relation between acid reflux in the proximal oesophagus and cardiorespiratory events in sleeping infants. Eur. J. Pediatrics 151, 208–212 (1992).
Harrington, C., Kirjavainen, T., Teng, A. & Sullivan, C. E. Altered autonomic function and reduced arousability in apparent life-threatening event infants with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 165, 1048–1054 (2002).
Walter, L. M. Back to Sleep or Not: The effect of the supine position on pediatric osa: sleeping position in children with osa: sleeping position in children with osa. Sleep Med. 37, 151–159 (2017).
Joosten, S. A., O’Driscoll, D. M., Berger, P. J. & Hamilton, G. S. Supine position related obstructive sleep apnea in adults: pathogenesis and treatment. Sleep. Med. Rev. 18, 7–17 (2013).
Coutier, L., Guyon, A., Reix, P. & Franco, P. Impact of prone positioning in infants with pierre robin sequence: a polysomnography study. Sleep. Med. 54, 257–261 (2019).
Orr, W. C. et al. Effect of sleep state and position on the incidence of obstructive and central apnea in infants. Pediatrics (Evanst.) 75, 832–835 (1985).
Berry, R. B., Quan, S. F., abreu, A. R., Bibbs, M. L. & DelRosso, L. The Aasm Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Spesifications, Version 2.6, Vol. 2.6 (American Academy of Sleep Medicine, 2020).
Garrick, W. D., Turkka, K., Catherine, B., Chris, S. & Karen, A. W. Site and mechanics of spontaneous, sleep-associated obstructive apnea in infants. J. Appl. Physiol. 89, 2453–2462 (2000).
Ginsburg, D. et al. Etiologies of apnea of infancy. Pediatr. Pulmonol. 55, 1495–1502 (2020).
May, J. G. et al. Systematic review of endoscopic airway findings in children with gastroesophageal reflux disease. Ann. Otol. Rhinol. Laryngol. 120, 116–122 (2011).
Sharma, P. B., Baroody, F., Gozal, D. & Lester, L. A. Obstructive sleep apnea in the formerly preterm infant: an overlooked diagnosis. Front. Neurol. 2, 73 (2011).
Katz, E. S., Mitchell, R. B. & D’Ambrosio, C. M. Obstructive sleep apnea in infants. Am. J. Respir. Crit. Care Med. 185, 805–816 (2012).
Qubty, W. F., Mrelashvili, A., Kotagal, S. & Lloyd, R. M. Comorbidities in infants with obstructive sleep apnea. J. Clin. Sleep. Med. 10, 1213–1216 (2014).
Huang, Y.-S., Paiva, T., Hsu, J.-F., Kuo, M.-C. & Guilleminault, C. Sleep and breathing in premature infants at 6 months post-natal age. BMC Pediatrics 14, 303–303 (2014).
Guilleminault, C. et al. Mixed and obstructive sleep apnea and near miss for sudden infant death syndrome: 2. comparison of near miss and normal control infants by age. Pediatrics 64, 882–891 (1979).
Ramgopal, S., Kothare, S. V., Rana, M., Singh, K. & Khatwa, U. Obstructive sleep apnea in infancy: a 7-year experience at a pediatric sleep center. Pediatr. Pulmonol. 49, 554–560 (2014).
Ratanakorn, W. et al. The maturation changes of sleep-related respiratory abnormalities in infants with laryngomalacia. J. Clin. Sleep. Med. 17, 767–777 (2021).
McNamara, F. & Sullivan, C. E. Obstructive sleep apnea in infants: relation to family history of sudden infant death syndrome, apparent life-threatening events, and obstructive sleep apnea. J. Pediatr. 136, 318–323 (2000).
Pereira, K. D. Body position and obstructive sleep apnea in 8–12-month-old infants. 72, 897-900 (2008).
Robison, J. G., Wilson, C., Otteson, T. D., Chakravorty, S. S. & Mehta, D. K. Analysis of outcomes in treatment of obstructive sleep apnea in infants. Laryngoscope 123, 2306–2314 (2013).
Moon, R. Y., TASK FORCE ON SUDDEN INFANT DEATH SYNDROME. SIDS and Other Sleep-Related Infant Deaths: Evidence Base for 2016 Updated Recommendations for a Safe Infant Sleeping Environment. Pediatrics 138, e20162938 (2016).
Gilbert, R., Salanti, G., Harden, M. & See, S. Infant Sleeping Position and the Sudden Infant Death Syndrome: Systematic Review of Observational Studies and Historical Review of Recommendations from 1940 to 2002. Int. J. Epidemiol. 34, 874–887 (2005).
Grigg-Damberger, M. et al. The visual scoring of sleep and arousal in infants and children. J. Clin. sleep. Med. 3, 201–240 (2007).
Paul, G. R. et al. What are the factors affecting total sleep time during video polysomnography in infants? Am. J. Perinatol. 39, 853–860 (2022).
Ellingson, R. J. & Peters, J. F. Development of eeg and daytime sleep patterns in normal full-term infant during the first 3 months of life: longitudinal observations. Electroencephalogr. Clin. Neurophysiol. 49, 112–124 (1980).
Crowell, D. H., Kapuniai, L. E., Boychuk, R. B., Light, M. J. & Hodgman, J. E. Daytime sleep stage organization in three-month-old infants. Electroencephalogr. Clin. Neurophysiol. 53, 36–47 (1982).
Orem, J. & Anderson, C. A. Diaphragmatic activity during rem sleep in the adult cat. J. Appl. Physiol. (Bethesda, Md: 1985) 81, 751–760 (1996).
Duara, S., Silva Neto, G. & Claure, N. Role of respiratory muscles in upper airway narrowing induced by inspiratory loading in preterm infants. J. Appl. Physiol. (Bethesda, Md: 1985) 77, 30–36 (1994).
van Leuteren, R. W., de Waal, C. G., Hutten, G. J., de Jongh, F. H. & van Kaam, A. H. Transcutaneous monitoring of diaphragm activity as a measure of work of breathing in preterm infants. Pediatr. Pulmonol. 56, 1593–1600 (2021).
Acknowledgements
As authors, we extend our gratitude to the staff of the Pediatric Sleep Study Center at Helsinki University Hospital for their contributions to this study. This work was supported by grants from the Foundation for Pediatric Research in Finland.
Funding
This study was supported by grants from the Foundation for Pediatric Research in Finland as a group grant to TK (#160268, #190140, #200184), and the Finnish Foundation for Pediatric Research supported writing of this as a grant for H-LK (#180025). Open Access funding provided by University of Helsinki including Helsinki University Central Hospital.
Author information
Authors and Affiliations
Contributions
T.K. was clinically responsible for the patients, scored and analyzed PSG studies, provided technical knowledge, and contributed to statistical analysis. H.-L.K. collected patient’s medical record data and did most of the statistical analysis. H.-L.K. and T.K. designed the study project and drafted and finalized the manuscript. Both authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kukkola, HL., Kirjavainen, T. Obstructive sleep apnea is position dependent in young infants. Pediatr Res 93, 1361–1367 (2023). https://doi.org/10.1038/s41390-022-02202-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02202-9