Abstract
Background
Sodium fluctuations in very preterm neonates and their neurodevelopmental consequences are not well described.
Methods
We assessed the changes in plasma sodium and glucose in the first days of life in very preterm neonates and studied the association of glucose-corrected plasma sodium fluctuations on neurodevelopmental outcomes. We included 147 consecutive neonates born before 29 weeks of gestation in our center and retrospectively obtained plasma sodium, glucose, and glucose-corrected sodium levels. Neurodevelopmental assessment was obtained from the Canadian Neonatal Follow-Up Network.
Results
Mean ± standard deviation of plasma sodium changes within the first 10 days of life were 16.2 ± 6.0, 14.8 ± 5.3, and 11.1 ± 5.2 mmol/l in neonates born ≤25, 25–26, and 26–27 weeks of gestation, respectively (p < 0.001). Non-steroidal anti-inflammatory drug administration was associated with larger plasma sodium fluctuation. Eighty-six percent had a known neurological status at 18 months. Higher fluctuations in glucose-corrected plasma sodium were associated with death or neurodevelopmental impairment at 18 months corrected age (B = 3.19, 95% CI [1.24, 5.14]), and this association remained after adjustment for gestational age (B = 2.1, 95% CI [0.16, 4.04]).
Conclusions
Neonates born very preterm show fluctuations in glucose-corrected plasma sodium during the first days of life, which may increase the risk of death or developmental impairment.
Impact
-
Risk factors and neurodevelopmental consequences of plasma sodium changes in early neonatal life of preterm infants are not well characterized.
-
This study shows for the first time that glucose-corrected plasma sodium fluctuations within the first days of life are more severe in preterm infants receiving non-steroidal anti-inflammatory drugs (NSAIDs) and are associated with death or neurodevelopmental impairment at 18 months corrected age.
-
Large plasma sodium and glucose fluctuations should be expected more often in preterm infants receiving NSAIDs and should be avoided.
Similar content being viewed by others
Introduction
For infants born extremely preterm (<28 weeks gestational age (GA)), the first days of extra-uterine life are associated with large fluctuations in electrolyte levels including plasma sodium levels resulting from extra-renal water and electrolyte losses and tubular immaturity, leading to difficult fluid and electrolyte management.1 Previous research has shown large plasma sodium changes, with rapidly occurring hypernatremia and hyponatremia, during the first days of life in neonates born very or extremely preterm.2,3 In the longer term, changes in neonatal plasma sodium have also been shown to be associated with bronchopulmonary dysplasia (BPD)4 and alterations in neurodevelopment.5,6
Preterm neonates are at increased risk of both hyperglycemic and hypoglycemic episodes,7 which may also alter neurodevelopment.8 In order to maintain plasma osmolality, plasma sodium levels are finely regulated in response to hyperglycemia.9 Plasma osmolality is therefore influenced by both plasma glucose and sodium levels. The brain is vulnerable to changes in plasma osmolality, with osmolar changes leading to changes in brain volume.10 Whether plasma osmolality, reflected by sodium levels corrected for blood glucose levels, is associated with neurodevelopment in infancy, or whether the association of plasma sodium changes and neurodevelopmental outcomes is simply the reflection of the adverse effect of poor blood glucose control on the immature brain, remain to be assessed.
In this study, we hypothesized that fluctuations of plasma sodium corrected for blood glucose during the first days of life in preterm neonates would be more severe in the most prematurely born infants and that the administration of drugs interfering with water and sodium metabolism at the kidney level, such as non-steroidal anti-inflammatory drugs (NSAIDs), may be associated with larger variations in plasma sodium. Greater fluctuations would be associated with an increased risk of death and neurodevelopmental impairment at 18 months’ corrected age (CA).
Methods
Study design and population
This is a retrospective observational cohort study of all infants born preterm ≤28 weeks’ GA between June 1, 2017 and December 31, 2018 at the CHU Sainte Justine neonatal intensive care unit, or admitted there within 48 h of life. Infants deceased within the first 10 days of life were excluded from the study. Ethical approval for this study was obtained from the CHU Sainte-Justine research ethics committee (approval number 2020-2691). Written informed consent had been obtained from all participating families to be part of the Canadian Neonatal Follow-Up Network research database, which allows for retrospective analyses upon ethics approval. Access to medical charts was granted by the director of medical affairs of CHU Sainte-Justine, as per provincial regulations.
Plasma sodium and glucose measurements
In order to obtain daily plasma sodium and glucose levels from day 1 to day 30 of life, we retrospectively collected all plasma sodium and glucose values available from the hospital’s biochemistry department for these dates. When more than one value was available for a single day, we used the median of these values. We found that plasma sodium and glucose-corrected plasma sodium variability was maximal in the 10 days following birth. We, thus, specifically studied the determinants and possible consequences of high glucose-corrected sodium changes occurring during the first 10 days of life in preterm neonates. From day 1 to day 10 of life, we obtained 1818 daily plasma sodium and 1803 daily glucose values. When no value was available for the day, we used a linear extrapolation to estimate the daily value from the previous and the next available values. From day 1 to day 10 of life, 302 plasma sodium values and 317 plasma glucose values were extrapolated. Sodium and glucose fluctuations were defined by the difference between the maximal value and the minimal value recorded for each individual. Plasma sodium and glucose were obtained from capillary blood gas measurements (ABL800, Radiometer, Mississauga, ON, Canada). Plasma sodium was corrected for glucose using the Katz formula:9
Some of the most extreme values of measured plasma sodium and glucose were outliers due to measurement errors (i.e., inaccurate blood sampling from the catheter). In such cases, repeat measurements were made to either confirm the outlier value or to provide a corrected value. The use of median values allowed us to avoid taking into account unconfirmed outliers.
18-month outcomes
As part of standardized follow-up care, all included preterm children were seen at 18–21 months’ CA for neurodevelopmental assessment as per the Canadian Neonatal Follow-Up Network protocol.11 Death after neonatal discharge was also ascertained through chart review or contact to families. At the 18-month visit, surviving children underwent a neurological examination by a trained pediatrician and developmental assessment with the Bayley Scales of Infant and Toddler Development, third edition, a standardized norm-referenced instrument that yields cognitive, language, and motor composite scores (mean 100, standard deviation 15). Medical charts were reviewed for auditory and visual function based on audiology and ophthalmology reports.
For the current study, we used a composite outcome of death at 18 months’ CA or neurodevelopmental impairment as these are competing events. Neurodevelopmental impairment was defined as the presence of any of the following: cerebral palsy, hearing loss requiring amplification, blindness, and a Bayley score ˂85. If the child was untestable due to significant developmental delay as per clinician’s judgment, that child was also considered to have neurodevelopmental impairment.
When unavailable for on-site visit, parents were contacted by phone to ensure vital status at 18 months CA.
Other variables of interest
Maternal and neonatal data were obtained from the Canadian Neonatal Network database and from medical charts. The presence and severity of BPD in the preterm group were defined using the classification from Jobe et al.12 Moderate-to-severe BPD was defined by oxygen supplementation requirement, continuous positive airway pressure or mechanical ventilation at 36 weeks postmenstrual age. We also used a composite outcome of death at 36 weeks postmenstrual age or BPD, as they are competing events.
We used the Score for Neonatal Acute Physiology (SNAP) to account for severity at birth.13 Acute kidney injury was defined by an increase in serum creatinine of at least 0.2 mg/dl (17 µmol/l) per day from a previous lower value, or by a serum creatinine level higher than 1.5 mg/dl (133 µmol/l).
Data analysis
Results are shown as means (standard deviation), medians (interquartile range) or n (%). Unadjusted comparisons between 3 groups of decreasing GA were performed using the Kendall test (continuous variables) or the Cochran–Armitage test (categorical variables). Associations between sodium or glucose fluctuations and composite outcomes of death or neurodevelopmental impairment, and death or BPD, were studied using linear models for variables in order to obtain estimates, 95% confidence intervals and p values. p Values <0.05 were considered statistically significant. The normality of residuals was assessed visually and using the Shapiro–Wilk test. All analyses were performed using R version 3.6.0 (International Open Source collaborative).14
Results
Population characteristics
There were 169 infants born during the study period. Neonates who died before 11 days of life (n = 22, including 15 with a GA <25 weeks) were excluded from the study. Thirteen children died between 11 days of life and the 18-month CA visit. At 18 months’ CA, neurodevelopmental assessment was available for 114 (85%) children, missing data being mainly due to COVID-19 pandemic restrictions. Table S1 provides the characteristics of patients with and without missing data at 18 months’ CA.
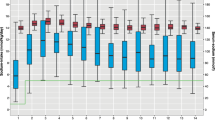
Characteristics of the cohort, according to GA, are provided in Table 1. Body weight changes were characterized by an initial decrease, followed by a rapid increase in birth weight. Changes in body weight were similar across all groups of GA at birth, although initial weight loss tended to be more severe in the <25 weeks GA group (Fig. 1). Plasma urea and creatinine levels tended to decrease with time, irrespective of GA. Plasma urea changes within the first month of life were more severe in those with a lower GA (Fig. 2).
Each point represents the mean daily value; when multiple measurements were performed in a single day, the median value was kept. When no measurement was obtained, the daily value was extrapolated from linear interpolation of the two closest values. Error bars indicate the 95th confidence interval for the mean.
Plasma sodium and glucose changes in the neonatal period according to GA
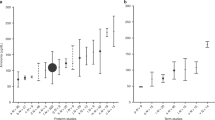
The first assessment of plasma sodium was similar across GA groups. Plasma sodium levels showed high variability immediately after birth in preterm neonates (Fig. 3). We found an increase in plasma sodium levels in the first 2 days after birth, followed by a rapid decrease in plasma sodium that was larger in neonates born with a lower GA. We found large variations in plasma glucose levels that were more severe in those with a lower GA at birth. Glucose-corrected plasma levels also showed increased variability with decreasing GA.
Each point represents the mean daily value; when multiple measurements were performed in a single day, the median value was kept. When no measurement was obtained, the daily value was extrapolated from linear interpolation of the two closest values. Plasma sodium and corrected sodium are shown as the mean daily change from baseline level (defined as the first plasma sodium measurement after birth), while glucose is shown as the mean daily absolute value. Error bars indicate the 95th confidence interval for the mean. Corrected sodium is calculated according to Katz.
Determinants of sodium and glucose fluctuations within the first 10 days of life and association with neonatal outcomes
Both glucose-corrected sodium and glucose fluctuations were more severe in the lowest GA group. More severe glucose-corrected sodium fluctuations were associated with a more severe neonatal course, as indicated by an association with markers of neonatal severity (SNAP, BPD). These associations did not persist, however, after adjustment for GA. Use of NSAIDs, but not acute kidney injury, was associated with increased glucose-corrected plasma sodium fluctuations, independently of GA. Glucose-corrected sodium fluctuations were also associated with severe intraventricular hemorrhage, independently of GA (Table 2).
Early sodium and glucose fluctuations and association with 18-month neurodevelopmental outcomes
Plasma glucose, sodium, and glucose-corrected sodium fluctuations during the first 10 days of life were associated with an increased occurrence of death or neurodevelopmental impairment at 18 months’ CA, and this association remained after adjustment for GA (Table 3).
Discussion
In this study, we show that neonates born preterm ≤28 weeks’ GA experience large fluctuations in plasma glucose and sodium levels after birth. These changes appear to be maximal in the 10 days following birth, and in those with the lowest GA. Changes in glucose-corrected plasma sodium levels were associated with an increased rate of severe intraventricular hemorrhage. Furthermore, both glucose and glucose-corrected plasma sodium fluctuations within the first 10 days of life were associated with death or neurodevelopmental impairment at 18 months’ CA, and these associations remained after adjustment for GA.
It was previously reported that water and sodium handling are altered in the extremely preterm neonate, and especially so in the first days of life. Extremely preterm infants show a prolonged period of high urinary sodium losses that require increased sodium intake to maintain homeostasis.15 In addition, sodium intakes are difficult to control, due to numerous sources of inadvertent sodium intake in the neonatal care unit, including through carrier solutions.1 Inability to maintain sodium homeostasis and difficulty to control sodium intake result in large fluctuations in plasma sodium within the first days of life in premature infants, which were previously reported in different settings.2,3 In our study, we also found large fluctuations in plasma sodium levels, that were more severe when GA was lower, similarly to previous research.3
The administration of NSAIDs in the first days of life for patent ductus arteriosus was associated with an increased risk of acute kidney injury, as previously reported,16 but also with increased plasma sodium fluctuations. NSAIDs promote water and sodium retention by inhibiting renal chloride reabsorption and increasing circulating levels of aldosterone and vasopressin.17 Ibuprofen, which was the most commonly used NSAID in our study, has previously been associated with hyponatremia when administered for patent ductus arteriosus.18 Special care should thus be given to plasma sodium levels in infants receiving NSAIDs for patent ductus arteriosus.
Rapid changes in plasma sodium, leading to changes in plasma osmolality, have been associated with neurological damage.19,20 We found that plasma sodium fluctuations were associated with an increased risk of severe intraventricular hemorrhage and that this association remained after adjustment for GA. These results are in line with previous research, which found that hypernatremia,21 hyponatremia, and sodium fluctuations22 were associated with the risk of intraventricular hemorrhage in preterm infants. We, thus, aimed to investigate the possible adverse effect of early plasma sodium fluctuations on brain development in preterm infants. Baraton et al. previously reported that large changes in plasma sodium levels occurring during the first month of life were associated with impaired functional outcomes at 2 years, after adjustment for GA.6 Howell et al. found that hypernatremia, defined by the occurrence of a plasma sodium level above 145 mmol/l, was associated with lower fine motor scores at 18 months’ CA.23 These two studies, however, did not account for plasma glucose levels, which also contribute to effective osmolality. In our study, we corrected plasma sodium levels for glucose levels, using the Katz formula,9 and found an association between the fluctuation of glucose-corrected plasma sodium levels during the first 10 days of life (when plasma sodium changes were identified as maximal) with neurodevelopment at 18 months’ CA, that remained after adjustment for GA and for the SNAP severity score.
In contrast, while we found an association between plasma-corrected sodium fluctuations and BPD, this association did not persist after adjustment for GA.
We found that early fluctuations in glucose levels were also independently associated with both a lower GA and suboptimal neurological outcomes, in line with a previous study that reported an association of neonatal hyperglycemia with impaired neurodevelopmental outcomes in extremely preterm infants.24 This highlights the necessity to correct plasma sodium levels for glucose levels when attempting to determine whether acute changes in plasma sodium may lead to adverse neonatal outcomes.
Our study has limitations. First, we could not obtain a neurodevelopmental assessment for all included infants in our study. This was mainly due to the COVID-19 pandemic-related travel and clinical restrictions; indeed, the assessment performed at 18 months’ CA could not be performed on a number of infants who should have been evaluated after March 2020. Nevertheless, we were able to obtain neurodevelopmental status for the majority of the infants included in the study. Second, we did not record daily intakes of electrolytes. Indeed, in the early neonatal period, a large proportion of sodium intake is inadvertently provided by various sources1 (catheter flushes, arterial blood pressure measurements, IV drugs including antibiotics…) making a reliable collection of this data impossible in this retrospective study. Third, a small number of patients n = 22 (15%) had missing data for NSAID use. One can reasonably presume that these infants (mostly 27–28 weekers) did not receive NSAID.
A strength of this study is the use of the Katz formula, which is largely used in clinical practice, to correct sodium values for blood glucose levels. Glucose levels were most of the time measured in the same sample as sodium samples, although when multiple measurements were performed in a single day, sodium and glucose were not necessarily measured contemporaneously. Hence, the use of both median glucose and sodium values allows to provide an approximation of daily glucose and sodium control, outside of extreme and possibly inaccurate fluctuations. We found a large variability of both plasma sodium and glucose levels within the first days of life in preterm neonates, and more specifically a high rate of hyperglycemia that was more severe in those with the lowest GA. We did not measure plasma osmolality. Measured plasma osmolality accounts for both effective osmoles (including sodium and glucose) and ineffective osmoles, which can freely cross cellular membranes and thus do not contribute to intracellular hydration. We show in this study large physiological variations in plasma urea within the first days of life, that is more severe in those born preterm. Urea is an ineffective osmole, that does not participate in an osmolar imbalance between the extracellular and the intracellular mediums. Hence, the use of the Katz formula to correct sodium levels for plasma glucose likely represents a more accurate estimation of effective plasma osmolality in this population and allows to estimate the deleterious effect of osmolar changes per se on neurological outcomes in very preterm infants.
This study demonstrates that plasma sodium fluctuations occurring within the first 10 days of life in very preterm infants are more severe in infants with a lower GA, those receiving NSAIDs, and are not solely related to changes in blood glucose. Large plasma sodium fluctuations may increase the risk of death or developmental impairment. These results have consequences in clinical practice. Indeed, while controlling for blood glucose and plasma sodium is a challenge, especially in the most preterm neonates, due to inadequate fluid and electrolyte homeostasis and inadvertent sodium intake, especially in those receiving NSAIDs, it may be of major importance for optimizing the neurodevelopmental outcomes of infants born extremely preterm.
Data availability
Raw data are available upon reasonable request to the corresponding author.
References
Eibensteiner, F. et al. ELBW infants receive inadvertent sodium load above the recommended intake. Pediatr. Res. 88, 412–420 (2020).
Späth, C., Sjöström, E. S., Ahlsson, F., Ågren, J. & Domellöf, M. Sodium supply influences plasma sodium concentration and the risks of hyper- and hyponatremia in extremely preterm infants. Pediatr. Res. 81, 455–460 (2017).
Monnikendam, C. S. et al. Dysnatremia in extremely low birth weight infants is associated with multiple adverse outcomes. J. Perinatol. 39, 842–847 (2019).
Rocha, G., Ribeiro, O. & Guimarães, H. Fluid and electrolyte balance during the first week of life and risk of bronchopulmonary dysplasia in the preterm neonate. Clinics 65, 663–674 (2010).
Barnette, A. R., Myers, B. J., Berg, C. S. & Inder, T. E. Sodium intake and intraventricular hemorrhage in the preterm infant. Ann. Neurol. 67, 817–823 (2010).
Baraton, L. et al. Impact of changes in serum sodium levels on 2-year neurologic outcomes for very preterm neonates. Pediatrics 124, e655–e661 (2009).
Galderisi, A. et al. Continuous glucose monitoring in very preterm infants: a randomized controlled trial. Pediatrics 140, e20171162 (2017).
Boscarino, G. et al. Neonatal hyperglycemia related to parenteral nutrition affects long-term neurodevelopment in preterm newborn: a prospective cohort study. Nutrients 13, 1930 (2021).
Katz, M. A. Hyperglycemia-induced hyponatremia-calculation of expected serum sodium depression. N. Engl. J. Med. 289, 843–844 (1973).
Verbalis, J. G. Brain volume regulation in response to changes in osmolality. Neuroscience 168, 862–870 (2010).
Synnes, A. et al. Determinants of developmental outcomes in a very preterm Canadian cohort. Arch. Dis. Child. Fetal Neonatal Ed. 102, F235–F234 (2017).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729 (2001).
Richardson, D. K., Gray, J. E., McCormick, M. C., Workman, K. & Goldmann, D. A. Score for Neonatal Acute Physiology: a physiologic severity index for neonatal intensive care. Pediatrics 91, 617–623 (1993).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2019).
Segar, J. L., Grobe, C. C. & Grobe, J. L. Maturational changes in sodium metabolism in periviable infants. Pediatr. Nephrol. https://doi.org/10.1007/s00467-021-05119-3 (2021).
Van Overmeire, B. et al. Prophylactic ibuprofen in premature infants: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 364, 1945–1949 (2004).
Knights, K. M., Mangoni, A. A. & Miners, J. O. Non-selective nonsteroidal anti-inflammatory drugs and cardiovascular events: is aldosterone the silent partner in crime? Br. J. Clin. Pharmacol. 61, 738–740 (2006).
Heo, M. J., Lee, O. S. & Lim, S. C. Comparative evaluation for the use of oral ibuprofen and intravenous indomethacin in Korean infants with patent ductus. Arch. Pharm. Res. 35, 1673–1683 (2012).
Pollock, A. S. & Arieff, A. I. Abnormalities of cell volume regulation and their functional consequences. Am. J. Physiol. 239, F195–F205 (1980).
Sterns, R. H. Disorders of plasma sodium-causes, consequences, and correction. N. Engl. J. Med. 372, 55–65 (2015).
Dalton, J., Dechert, R. E. & Sarkar, S. Assessment of association between rapid fluctuations in serum sodium and intraventricular hemorrhage in hypernatremic preterm infants. Am. J. Perinatol. 32, 795–802 (2015).
Lim, W.-H. et al. Hypernatremia and grade III/IV intraventricular hemorrhage among extremely low birth weight infants. J. Perinatol. 31, 193–198 (2011).
Howell, H. B. et al. The impact of hypernatremia in preterm infants on neurodevelopmental outcome at 18 months of corrected age. Am. J. Perinatol. https://doi.org/10.1055/s-0040-1716845 (2020).
Zamir, I. et al. Neonatal hyperglycaemia is associated with worse neurodevelopmental outcomes in extremely preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 106, 460–466 (2021).
Funding
This work was supported by a Fonds de recherche du Québec – Santé (FRQS)/Fondation des Étoiles fellowship award to A.F.
Author information
Authors and Affiliations
Contributions
A.-S.G., T.M.L., A.V., A.-A.M., J.V., A.C., and F.L. collected the data. AF performed data analysis. A.-S.G., T.M.L., A.M.N., and A.F. wrote the first version of the manuscript. A.V., A.-A.M., J.V., A.C., and F.L. participated in the interpretation of the results. A.F. supervised the study. All authors revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Written informed consent had been obtained from all participating families to be part of the Canadian Neonatal Follow-Up Network research database, which allows for retrospective analyses upon ethics approval.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gervais, AS., Luu, T.M., Viennet, A. et al. Neurodevelopmental consequences of early plasma sodium changes in very preterm infants. Pediatr Res 92, 1350–1356 (2022). https://doi.org/10.1038/s41390-022-02164-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02164-y
This article is cited by
-
Sulindac selectively induces autophagic apoptosis of GABAergic neurons and alters motor behaviour in zebrafish
Nature Communications (2023)
-
The elusive biomarker
Pediatric Research (2022)