Abstract
Background
Angiotensin-converting enzyme 2 (ACE2) is the receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which causes COVID-19. Viral entry requires ACE2 and transmembrane protease serine 2 (TMPRSS2). Transcriptomic studies showed that children display lower ACE2 than adults, though gene expression levels do not always correlate with protein levels. We investigated the effect of age on ACE2 and TMPRSS2 protein expression in alveolar type II (AT2) cells in the lungs of children compared to adults. We also analysed the ratio of Ang-(1–7) to Ang II as a surrogate marker of ACE2 activity in the subjects’ lung parenchyma.
Methods
Ang II and Ang-(1–7) levels and ACE2 and TMPRSS2 protein expression were measured by radioimmunoassay and immunohistochemistry, respectively.
Results
The amount of ACE2-expressing AT2 cells and ACE2 protein content were lower in children than in adults. Ang II levels were higher in children compared to adults and inversely correlated with the amount of ACE2-expressing AT2 cells. Children presented lower Ang-(1–7)/Ang II ratio than adult suggesting lower ACE2 activity in children. TMPRSS2 protein expression was not influenced by age.
Conclusions
These results expand on previous transcriptomic studies and may partially explain the low susceptibility of children to SARS-CoV-2 infection.
Category of study
Clinical original research
Impact
-
Children display lower ACE2 protein content and activity compared to adults.
-
Ang II levels were higher in children compared to adults and inversely correlated with the amount of ACE2-expressing AT2 cells
-
TMPRSS2 protein expression was not influenced by age.
-
These results expand on previous transcriptomic studies and may partially explain the low susceptibility of children to SARS-CoV-2 infection.
Similar content being viewed by others
Introduction
Angiotensin-converting enzyme 2 (ACE2) is a key enzyme in the renin-angiotensin system (RAS) responsible for balancing the pressor arm of the RAS by catalysing the conversion of angiotensin (Ang) II into the protective component Ang-(1–7)1. In the lungs, ACE2 is primarily expressed in alveolar type II (AT2) cells2,3,4,5, which are key to maintaining lung homoeostasis. ACE2 also acts as the receptor for the type 2 coronavirus that causes severe acute respiratory syndrome (SARS-CoV-2), the aetiological agent of coronavirus disease 2019 (COVID-19). The spike protein on SARS-CoV-2 binds ACE2 and is primed by transmembrane protease serine 2 (TMPRSS2), allowing the virus entry into the host cell6.
Children have higher resistance to COVID-19 than adults7. This diminished susceptibility may be related to ACE2 and TMPRSS2 levels. Several reports have shown that children display lower ACE2 and TMPRSS2 gene expression than adults8,9,10. Gene expression do not always correlate with protein levels due to protein post-transcriptional modifications or low mRNA stability. To our knowledge, there is only one report on ACE2 protein expression in AT2 cells in children5, though it was used two different cohorts, different brands of ACE2 antibody for each cohort and a small number of samples (4 and 10 samples in cohort 1 and 2, respectively). They found no difference in ACE2 protein expression by age. To further confirm the effect of age on ACE2 and TMPRSS2, we investigated ACE2 and TMPRSS2 protein expression in AT2 cells in the lungs of children compared to adults. We also analysed Ang II and Ang-(1–7) levels in the subjects’ lung parenchyma to employ the ratio of Ang-(1–7) to Ang II as a surrogate marker of ACE2 activity.
Methods
Human lung samples
This study was an observational retrospective study of de-identified material; informed consent was not required. Our Institutional Review Board (Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires and Hospital del Tórax) approved all protocols. Lung parenchymal samples from children (1 to 18 years of age, n = 21), adult subjects between 20 and 60 years of age (n = 26) and subjects 60 years of age and older (n = 29) who underwent resection (segmentectomy or lobectomy) were obtained from the archives of the Pathology Service of the Hospital Provincial del Tórax. Tissues were only used if they were characterised as non-diseased, that is, areas of the tissue that were not involved in patient’s underlying medical conditions (i.e., neoplasia, infections, bronchiectasis). The same sample was analysed for Ang II and Ang-(1–7) levels and ACE2 and TMPRSS2 protein expression.
Ang II and Ang-(1–7) levels measurement
Samples were dewaxed, rehydrated and homogenisd in acid ethanol (0.1 mmol/L HCl/80% ethanol) containing a mix of protease inhibitors. Homogenates were centrifuged at 20,000 × g for 30 min at 4 °C. The supernatant was subsequently lyophilised and angiotensin extraction and measurement by radioimmunoassay were performed as previously described11.
ACE2 and TMPRSS2 immunohistochemistry
ACE2 and TMPRSS2 protein expression were measured by immunohistochemistry performed on a Discovery Ultra VENTANA Systems (Roche) automated stainer. Samples were exposed to a rabbit polyclonal anti-ACE2 antibody (Abcam cat. ab15348, lot GR3333640-5, dilution 1:500) or a rabbit monoclonal anti-TMPRSS2 antibody (Abcam cat. ab92323, lot GR3344246-11, dilution 1:1500) together with a mouse monoclonal antibody TTF-1 (Cell Marque clone 8G7G3/1, lot 077115, dilution 1:500) to label AT2 cells. TTF-1 is the gold standard for identifying lung adenocarcinoma and it is known as a nuclear homeodomain transcription factor that is expressed in the thyroid, in the normal bronchiolar and alveolar epithelium as well as in pulmonary neoplasms, particularly in adenocarcinomas, or in the developing forebrain12. In the lung, TTF-1 is expressed in AT2 cells and Clara cells13. TTF-1-positive cells with cuboidal morphology along the alveolar septum were counted as AT2 cells. Antibody binding was visualised following incubation with the ultraView Universal Alkaline Phosphatase Red Detection Kit (Roche cat. 0785269814001) for ACE2 and TMPRSS2 and the ultraView Universal DAB Detection Kit (Roche cat. SAP 5269806001) for AT2 cells. Images were taken on an Olympus CX31 microscope. Percentages of ACE2- and TMPRSS2-expressing AT2 cells were measured in representative lung fields with sufficient cells to allow no fewer than 450 AT2 cells to be counted. ACE2 intensity was classified from +1 to +3 (+1, very weak; +2, moderate; +3, strong). Two independent, qualified pathologists specialising in the human lung, analysed ACE2 and TMPRSS2 staining in AT2 cells after being blinded to the patient’s condition, following the principles for reproducible tissue scores.
Statistical analysis
The data were analysed by a statistician (M.N., chair of Mathematics and Biostatistics at the Faculty of Pharmacy and Biochemistry, University of Buenos Aires) using the IBM SPSS Statistics 19 software. No differences by sex were found. Homogeneity of variances was determined by Levene’s test. The Kruskal–Wallis test was applied to non-normally distributed data. Subsequent post hoc analyses for significant results were performed with the Bonferroni test. The Spearman coefficient was calculated to analyse correlations. A probability level of 0.05 was considered significant.
Results
Children displayed significantly higher Ang II and Ang-(1–7) levels than adult subjects both below 60 years of age and 60 years of age and older (Fig. 1a, b, respectively). The ratio of Ang-(1–7) to Ang II, which acted as a surrogate marker of ACE2 activity, was thus lower in children compared to adults (P = 0.05) (Fig. 1c).
a Ang II and b Ang-(1–7) levels and c Ang-(1–7)/Ang II ratio in lung parenchyma of children (Ch; n = 21), adult subjects below 60 years of age (< 60 y; n = 26) and subjects 60 years of age and older (≥ 60 y; n = 29). The bottom and top of the box plots represent the 25th and 75th percentiles, respectively. The bands within the box indicate the median and the whiskers extending from both ends of the boxes the minimum and maximum values. P values are indicated in each graph (Kruskal–Wallis test).
We then evaluated ACE2- and TMPRSS2-expressing AT2 cells. TTF-1-positive cells with cuboidal morphology along the alveolar septum were counted as AT2 cells. Children exhibited fewer AT2 cells that express ACE2 compared to adult subjects both below 60 years of age and 60 years of age and older (P = 0.0001) (Fig. 2). There was no difference between children and adults in the number of AT2 cells that express TMPRSS2 (Fig. 3).
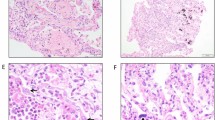
Percentage of ACE2-expressing type II alveolar (AT2) cells in the lung parenchyma of children (n = 21), adult subjects below 60 years of age (< 60 years; n = 26) and subjects 60 years of age and older (≥ 60 years; n = 29). The bottom and top of the box plots represent the 25th and 75th percentiles, respectively. The bands within the box indicate the median and the whiskers extending from both ends of the boxes the minimum and maximum values. Representative images of surgically resected lung tissue stained for TTF-1-positive (blue arrow), ACE2-positive (yellow arrow) or TTF-1/ACE2 double positive (red arrow) cells counterstained with haematoxylin (blue). Brown cells are TTF1-positive cells. Scale bar: 20 µm. * P = 0.0001 when compared to < 60 years and ≥ 60 years (Kruskal–Wallis).
Percentage of TMPRSS2-expressing AT2 cells in the lung parenchyma of children (n = 21), adult subjects below 60 years of age (< 60 years; n = 26) and subjects 60 years of age and older (≥ 60 years; n = 29). The bottom and top of the box plots represent the 25th and 75th percentiles, respectively. The bands within the box indicate the median and the whiskers extending from both ends of the boxes the minimum and maximum values. Representative images of surgically resected lung tissue stained for TTF-1-positive (blue arrow), TMPRSS2-positive (yellow arrow) or TTF-1/TMPRSS2 double positive (red arrow) cells counterstained with haematoxylin (blue). Brown cells are TTF1-positive cells. Scale bar: 20 µm.
There was a significant negative correlation between Ang II levels and the concentration of ACE2-expressing AT2 cells in children (r = −0.484; P = 0.035) and in adult subjects below 60 years of age (r = −0.408; P = 0.041). No correlation was found between Ang-(1–7) levels and the number of ACE2-expressing AT2 cells.
In addition to ACE2-expressing AT2 cells, ACE2 protein content in AT2 cells was also lower in children. As shown in Fig. 4a, children displayed the lowest ACE2 content in AT2 cells of all age groups (P < 0.0001). TMPRSS2 protein content was not different between children and adults (Fig. 4b).
a Percentage of subjects with ACE2 and b TMPRSS2 immunostaining intensity from +1 to +3 in type II alveolar cells of lung parenchyma of children (Ch), adults below 60 years of age (< 60) and adults 60 years of age and older (≥ 60). Children did not display +3 intensity of staining for both ACE2 and TMPRSS2. Lung parenchymal samples from children (1 to 18 years of age, n = 21), adult subjects between 20 and 60 years of age (n = 26) and subjects 60 years of age and older (n = 29) were analysed. Representative images of surgically resected lung tissue stained for ACE2 or TMPRSS2 protein (red, as indicated by black arrows) and AT2 cells (brown) counterstained with haematoxylin (blue). Brown cells are TTF1-positive cells. Scale bar: 20 µm. * P < 0.0001 when compared to < 60 and ≥ 60 (Kruskal–Wallis).
Discussion
Our study showed that children displayed higher lung Ang II levels and a lower Ang-(1–7)/Ang II ratio compared to adult subjects, suggesting lower ACE2 activity in children. Furthermore, the percentage of ACE2-expressing AT2 cells was lower in children than in adult subjects. In children, Ang II levels were inversely correlated with the percentage of AT2 cells that express ACE2 protein. In addition, we found that the AT2 cells present in the lungs of children contained less ACE2 protein content compared to adults.
Ang-(1–7) levels were also higher in children. Ang-(1–7) can be formed not only from Ang II but also from Ang I through the catalytic activity of neutral endopeptidase, neprilysin, thimet oligopeptidase and prolyl oligopeptidase. We disregard Ang-(1–7) generation from Ang-(1–9) because ACE2 protein expression was decreased in children in this study; thus, generation of Ang-(1–9) due to ACE2 activity may not constitute a significant source of Ang-(1–7).
We found that the levels of both AT2 cells that express ACE2 and ACE2 protein were lower in children than in adults. Similarly, children have been found to have significantly lower ACE2 gene expression in the upper (nasal) and lower (bronchial) airways compared to adults8,9,14, even in term and preterm newborns14. Comparable ACE2 expression levels were found in children and adults in two different cohorts, though different brands of antibodies for each cohort were employed and the number of samples from children was smaller compared to our study (4 and 10 samples in cohort 1 and 2, respectively, versus 21 samples in our study)5.
Considering that SARS-CoV-2 requires ACE2 to enter cells, it can be hypothesised that ACE2 levels may be related to COVID-19 infection rate and severity. However, it has been recently shown that ACE2-expressed cells in the alveolus of children and older adults demonstrated similar susceptibility to ex vivo infection of pseudoviral SARS-CoV-24. In contrast, Yonker et al15. reported that although nasopharyngeal viral load was highest in children in the first two days of symptoms and significantly higher than in hospitalised adults with severe disease, children had lower ACE2 expression. Nevertheless, additional factors such as the immune response may contribute to the explanation of the low susceptibility of children to SARS-CoV-216.
Regarding TMPRSS2, another key protein for the entry of SARS-CoV-2 into cells, we did not observe differences in the amount of TMPRSS2-expressing AT2 cells between children and adults, which is consistent with previous findings that AT2 cell expression of TMPRSS2 does not change with age4,17.
Our study was limited by a retrospective design, which is vulnerable to uncontrolled biases. While some of the resection samples were from people with cancer, we utilised healthy tissue adjacent to the diseased tissue; still, we cannot disregard the influence of cancer on the results.
In conclusion, our study demonstrated for the first time that levels of Ang II were higher, while its degradation was lower, in children compared to adults. Furthermore, ACE2 protein expression was lower in children and TMPRSS2 did not change with age. These results expand on previous transcriptomic studies and may partially explain the low susceptibility of children to SARS-CoV-2 infection.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Santos, R. A. S. et al. The renin-angiotensin system: going beyond the classical paradigms. Am. J. Physiol. Heart Circ. Physiol. 316, H958–H970 (2019).
Patel S. K. et al. Plasma ACE2 activity is persistently elevated following SARS-CoV-2 infection: implications for COVID-19 pathogenesis and consequences. Eur. Respir. J. 57, 2003730 (2021).
Zou, X. et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 14, 185–192 (2020).
Zhang Z. et al. Distinct disease severity between children and older adults with COVID-19: Impacts of ACE2 expression, distribution, and lung progenitor cells. Clin. Infect. Dis. 73,e4154–e4165 (2021).
Tao Y. et al. Preliminary analyses of scRNA sequencing and immunohistochemistry of children’s lung tissues indicate the expression of SARS‐CoV‐2 entry‐related genes may not be the key reason for the milder syndromes of COVID‐19 in children. Clin. Transl. Med. 11, e300 (2021).
V’kovski, P., Kratzel, A., Steiner, S., Stalder, H. & Thiel, V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2020 193 19, 155–170 (2020).
Wu, Z. & McGoogan, J. M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323, 1239–1242 (2020).
Saheb Sharif-Askari, N. et al. Airways expression of SARS-CoV-2 receptor, ACE2, and TMPRSS2 is lower in children than adults and increases with smoking and COPD. Mol. Ther. Methods Clin. Dev. 18, 1–6 (2020).
Bunyavanich, S., Do, A. & Vicencio, A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA 323, 2427–2429 (2020).
Wang, A. et al. Single-cell multiomic profiling of human lungs reveals cell-type-specific and age-dynamic control of SARS-CoV2 host genes. Elife 9, 1–28 (2020).
Cerniello, F. M., Silva, M. G., Carretero, O. A. & Gironacci, M. M. Mas receptor is translocated to the nucleus upon agonist stimulation in brainstem neurons from spontaneously hypertensive rats but not normotensive rats. Cardiovasc. Res 116, 1995–2008 (2020).
Miura K. et al. A novel strategy for the diagnosis of pulmonary high-grade neuroendocrine tumor. Diagnostics 11, 1945 (2021).
Ordóñez, N. G. Value of thyroid transcription factor-1 immunostaining in tumor diagnosis: a review and update. Appl. Immunohistochem. Mol. Morphol. AIMM 20, 429–444 (2012).
Heinonen S. et al. Nasal expression of SARS-CoV-2 entry receptors in newborns. Arch. Dis. Child. Fetal Neonatal Ed. 107, 95–97 (2021).
Yonker, L. M. et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune Responses. J. Pediatr. 227, 45–52.e5 (2020).
Zimmermann, P. & Curtis, N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch. Dis. Child. 106, 429–439 (2020).
Schuler B. A. et al. Age-determined expression of priming protease TMPRSS2 and localization of SARS-CoV-2 in lung epithelium. J. Clin. Invest. 131, e140766 (2021).
Acknowledgements
The technical assistance of Laura Iglesias and Ivana Rodriguez is greatly appreciated.
Funding
This work was supported by a grant from Agencia Nacional de Promoción Científica y Tecnológica [grant number IP COVID-19 893]. The funder was not involved in study design, conduct, data analysis, manuscript preparation or publication.
Author information
Authors and Affiliations
Contributions
Each author has met the authorship requirements in that each has significantly contributed to the interpretation of data, drafting the article or revising it critically for important intellectual content, and given final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, M.G., Falcoff, N.L., Corradi, G.R. et al. Effect of age on human ACE2 and ACE2-expressing alveolar type II cells levels. Pediatr Res 93, 948–952 (2023). https://doi.org/10.1038/s41390-022-02163-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02163-z
This article is cited by
-
Severe pediatric COVID-19: a review from the clinical and immunopathophysiological perspectives
World Journal of Pediatrics (2024)
-
Immune profiling of SARS-CoV-2 epitopes in asymptomatic and symptomatic pediatric and adult patients
Journal of Translational Medicine (2023)
-
Understanding COVID-19 in children: immune determinants and post-infection conditions
Pediatric Research (2023)
-
Preserved perception-action integration in adolescents after a COVID-19 infection
Scientific Reports (2023)
-
Analyzing the role of ACE2, AR, MX1 and TMPRSS2 genetic markers for COVID-19 severity
Human Genomics (2023)