Abstract
Background
Preterm birth is associated with higher risks of suboptimal neurodevelopment and cardiometabolic disease later in life. Altered maternal–fetal lipid supply could play a role in such risks. Our hypothesis was that very preterm infants born with very low birth weight (VLBW) have altered lipidome and apolipoprotein profiles, compared with term infants.
Methods
Seven mothers of VLBW infants born at <32 GA and 8 full-term mother–infant dyads were included. Cholesterol and triglycerides in lipoproteins were determined in maternal plasma and in the two blood vessels of the umbilical cord (vein (UV) and artery (UA)) following FPLC isolation. Apolipoprotein concentrations in lipoproteins and plasma lipidomic analysis were performed by LC-MS/MS.
Results
We found higher cholesterol and VLDL-cholesterol in UV and UA and lower apolipoprotein A-I in HDL2 in UV in preterm neonates. Phosphatidylcholine (PC) containing saturated and monounsaturated fatty acids and specific sphingomyelin species were increased in UV and UA, whereas PC containing docosahexaenoic acid (DHA) was reduced in UV of VLBW neonates.
Conclusions
Lower DHA-PC suggests a lower DHA bioavailability and may contribute to the impaired neurodevelopment. Altered HDL-2, VLDL, and sphingomyelin profile reflect an atherogenic risk and increased metabolic risk at adulthood in infants born prematurely.
Impact
-
Lower ApoA-I in HDL2, and increased specific sphingomyelin and phosphatidylcholine containing saturated and monounsaturated fatty acid could explain the accumulation of cholesterol in umbilical vein in VLBW preterm neonates.
-
Decreased phosphatidylcholine containing DHA suggest a reduced DHA availability for brain development in VLBW preterm infants.
-
Characterization of alterations in fetal lipid plasma and lipoprotein profiles may help to explain at least in part the causes of the elevated cardiovascular risk known in people born prematurely and may suggest that a targeted nutritional strategy based on the composition of fatty acids carried by phosphatidylcholine may be promising in infants born very early.
Similar content being viewed by others
Introduction
Preterm birth, defined as delivery prior to 37 weeks of gestation, is a major public health issue as it is associated with increased neonatal morbidity and mortality,1 and its prevalence is on the rise.2 Preterm birth abruptly interrupts maternal–fetal nutrient transfer at a time of tremendous growth (since a normal fetus triples its weight in the third trimester) and of utmost importance for the development of most fetal organs, particularly central nervous system.3 Moreover, long-term follow-up studies have demonstrated that infants born preterm and/or with intrauterine growth restriction (IUGR) are exposed to enhanced risk of impaired postnatal neurodevelopment4,5 and increased cardiovascular risk later in life.6
The lipoprotein profile in umbilical cord blood is influenced by fetal prematurity and undernutrition.7,8 Using an 1H-nuclear magnetic resonance-based metabolomic approach, we previously observed that lipoproteins, notably low-density lipoprotein (LDL) and very-low-density lipoprotein (VLDL), then high-density lipoprotein (HDL) were lower in umbilical cord vein and arterial (UV and UA, respectively) plasma of very low birth weight (VLBW) preterm, compared to term neonates.9 Studies in preterm neonates have largely demonstrated alterations in classical lipid parameters, such as plasma cholesterol, HDL cholesterol (HDL-c), LDL cholesterol (LDL-c), triglycerides (TGs), apolipoprotein A-I (ApoA-I), or apolipoprotein B (ApoB). Cholesterol level was higher in umbilical cord venous (UV) blood from near-term neonates (born between 35 and 36.6 weeks of gestation with a birth weight (BW) mean of 2400 g) than from infants born at full term, due to an increase in the LDL-c fraction.10 Moreover, plasma and LDL-c remains at high level in preschool children (5–7 years) born very preterm, compared with infants born at full term.11 By comparison to full-term infants, altered fatty acid profile and particularly lower long-chain polyunsaturated fatty acids (LCPUFA) have been observed in premature infants (born prior to 36 weeks of gestation)12 and particularly in small for gestational age (GA) preterm13 suggesting impaired fetal metabolism of bioactive complex lipids that may have deleterious effects on the neurodevelopment of these infants.
Although numerous studies have been carried out in preterm neonates,10,14 none has simultaneously explored the lipid and apolipoprotein profile in lipoprotein, and the plasma lipidome in both UV (which carries blood from placenta to fetus) and UA (which carries blood from fetus to placenta) in very preterm neonates (<32 weeks of gestation) with a VLBW (<1500 g).
Our working hypothesis was that the use of lipidomics combined with the simultaneous assessment of the two blood vessels of umbilical cord would highlight profound alterations in lipoprotein profile and lipidome in preterm, compared with term infants.
The objectives of this pilot study were to (1) determine the profiles of cholesterol and TG-associated lipoprotein particles in maternal blood and UV and UA blood, (2) quantify the apolipoproteins in lipoproteins, (3) characterize the modifications in plasma lipidome, particularly fatty acid composition in phospholipids, TG, cholesteryl ester, and other complex lipids in VLBW preterm and term neonates and their respective mothers, and (4) investigate the relationship between lipids such as cholesterol, TGs, and apolipoproteins in lipoproteins and other bioactive lipid metabolites using a lipidomic analysis in mother–infant dyads after either a full-term pregnancy or a very preterm delivery.
A better understanding of these early metabolic changes in infants born very prematurely should help develop novel strategies for primary prevention of impaired neurodevelopment and chronic diseases.
Materials and methods
Study design and subjects
The registered study (ClinicalTrials.gov Identifier: NCT00607061) was approved by the local medical ethical committee (Comité de protection des personnes (CPP) des Pays de la Loire) and performed in the Department of Obstetrics of the Hôpital Mère-et-Enfant at the University Hospital of Nantes. A written informed consent was obtained from every mother a few hours before delivery. The lipidomic investigation in this pilot study was conducted in a subset of a larger sample size study previously reported.9,15 The blood from UV and UA was immediately collected at the time of birth in preterm deliveries (n = 7) and in a control group of full-term deliveries (n = 8). Maternal venous blood was also collected at delivery for every neonate included to obtain insight into lipid maternal–fetal transfer in both groups. Very preterm infants with a GA <32 weeks and a BW of 1500 g were enrolled as defined as high risk for death and medical complication.16
The control group was selected with a GA over 37 weeks and uneventful pregnancy and delivery. No fetal pathology was revealed during pregnancy (Table 1). The sample size of the study is small due to the difficulty of recruiting mothers in the stressful time of premature birth and obtaining the blood samples from three sites (maternal vein, UV, and UA).
Lipid profiles
Isolation of lipoproteins
Lipoprotein fractions in plasma from all three sampling sites were separated using a Fast Protein Liquid Chromatography (FPLC) system based on the gel filtration property (AKTA FPLC SYSTEM®, GE Healthcare, Piscataway, NJ). A 100 µL aliquot of plasma was used to separate VLDL, IDL, LDL, HDL2, and HDL3 as previously described.17 Total cholesterol and TG concentrations were measured in plasma and in all lipoprotein fractions using colorimetric kits from Boehringer Mannheim GmbH (Mannheim, Germany). Then lipoprotein fractions (500 µL) were desalted and concentrated with 3 mL of 50 mmol/L ammonium bicarbonate buffer using a 5 kDa molecular mass cut-off filter (Amicon®, Merck-Millipore, Saint-Quentin-Fallavier, France) for apolipoprotein quantification.
Apolipoprotein quantification in lipoproteins
Apolipoproteins were quantified in concentrated lipoprotein fractions by liquid chromatography–tandem mass spectrometry (LC-MS/MS) as previously described.18,19 Apolipoproteins were quantified using trypsin proteolysis and the subsequent analysis of proteotypic peptides by LC-MS/MS. Samples were prepared with the ProteinWorks™ eXpress kit (Waters, Milford, MA), according to the manufacturer’s instructions. Synthetic labeled and unlabeled proteotypic peptides were provided by Thermo Scientific Biopolymers (Darmstadt, Germany). Stock solutions (1 mmol/L) were prepared in 50% acetonitrile containing 0.1% formic acid and stored at −20 °C until use. A mixed solution of unlabeled peptides was constituted and serially diluted in water to obtain seven standard solutions as previously validated.19 Labeled peptides (i.e., containing [13C615,N2] K or [13C615,N4] R in the C-terminal position) were used as internal standards (ISs). A mixed solution of ISs (35 µmol/L) was prepared and added to digestion buffer (ammonium bicarbonate, 50 mmol/L) to a final concentration of 1.75 µmol/L. Samples (40 µL) were incubated for 10 min at 80 °C in digestion buffer containing ISs (100 µL) and RapidGest detergent solution (7 mg/mL, 10 µL), reduced for 20 min at 60 °C with dithiothreitol (70 mmol/L, 20 µL), alkylated for 30 min at room temperature in the dark with iodoacetamide (142 mmol/L, 30 µL), and digested overnight at 37 °C (~16 h) with trypsin (7 mg/mL, 30 µL). Enzymatic digestion was stopped with 20% trifluoroacetic acid (5 µL). After 15 min at 45 °C, the precipitate was removed by centrifugation (15 min, 10 °C, 10,000 × g), and supernatants were cleaned on 30 mg Oasis HLB cartridges (Waters), which were conditioned (100% methanol; 1 mL), equilibrated (100% water; 1 mL), loaded (sample; ~200 µL), washed (5% methanol; 1 mL), and eluted (80% methanol; 500 µL). The eluates were dried under nitrogen (45 °C), reconstituted with 5% acetonitrile containing 0.1% formic acid (100 µL), and injected (10 µL) into the LC-MS/MS system. Analyses were performed on a Xevo® TQD mass spectrometer with an electrospray (ESI) interface and an Acquity H-Class® UPLC™ device (Waters). The optimized LC-MS/MS and “multiple reaction monitoring” parameters have been detailed previously.18,19
Lipidomic analysis in plasma
Lipids were extracted from plasma samples of maternal and UV and UA plasma and analyzed by liquid chromatography–high-resolution mass spectrometry (LC-HRMS as extensively described previously.20,21 Briefly, a mixture of internal standards was added to the plasma samples and to the quality control samples (QC). Lipids were extracted twice from the plasma using Bligh and Dyer method22 and centrifuged at 10,000 rpm for 10 min at 4 °C. The combined supernatants of lipids were transferred to a fresh 2-mL glass vial, dried under nitrogen, and reconstituted in 100 μL of an isopropanol/acetonitrile/H2O mixture (2:1:1, v:v:v). Lipid species analysis was performed on a Synapt™ G2 HDMS Q-TOF mass spectrometer, equipped with an ESI interface operating in the positive and negative ionization mode, combined to an Acquity H-Class® UPLC™ device (Waters). Samples and QC extracts were randomized and injected (5 µL) on the LC-HRMS device (Waters). The optimized LC-HRMS parameters have been detailed previously.20,21 Data acquisition and processing of mass spectrometry data, including peak detection, integration, alignment, and normalization, were achieved using the MassLynx® and MakerLynx® software (version 4.1, Waters Corporation). Lipid markers were selected from the detected features using an in-house lipidomic database of 180 lipid species built by the use of lipid standards, the exact mass measured, the elemental compositions with a mass error <5 ppm, the retention times, and the fragmentation patterns as earlier described.23 The relative standard deviation (RSD, %) was calculated for peak areas in the QC samples to highlight the repeatability of the analytical process. Selected lipid markers having a RSD value <30% were retained for multivariate analysis.20
The identification of lipids was performed using the LIPID MAPS database (LIPID Metabolites and Pathways Strategy, http://www.lipidmaps.org/) and based on the exact mass measured including adduct formed for the ionization.
Statistical analysis
Statistical analyses were performed with GraphPad Prism® version 8.01. All variables were compared between two independent groups, preterm vs. term groups, in maternal, UV, and UA plasma by Mann–Whitney U-test. Chi-square test was used to compare qualitative data in preterm and full-term babies. p Value correction based on the false discovery rate using the q value concept with a significance threshold set at q ≤ 0.05 was used for the lipidomic analysis.24
Pearson’s correlation was performed to assess correlations between total cholesterol or phosphatidylcholine (PC) (38:6) and GA, BW, and critical lipids (sphingomyelin (SM), PC, and fatty acids) in UV plasma. Results are expressed as means ± SD (standard deviation). Differences were considered significant at p < 0.05.
Results
A total of 15 mother–infant dyads, 8 full-term and 7 VLBW preterm neonates were enrolled. The body mass index of all the 15 mothers were <25 kg/m2. The 7 mothers with preterm neonates delivered before 32 weeks and some of the preterm babies were also suffering from IUGR, as shown by the lower percentile of BW (Table 1).
Cholesterol was increased in umbilical cord plasma of preterm neonates
Cholesterol concentration was increased in UV plasma in VLBW preterm compared to term infants, whereas no difference in circulating cholesterol was detected among mothers (Fig. 1a). Maternal and fetal plasma TG concentration were not different between VLBW and term neonates (Fig. 1b). To characterize lipoprotein profile, plasma from maternal blood and from cord blood was submitted to FPLC and VLDL, LDL-c, HDL-c, and TG were determined in maternal and cord plasma. Based on lipoprotein profile in adult,25 we identified four peaks that we characterized as VLDL-, LDL-, HDL2-, and HDL3-like lipoproteins, respectively. As shown in Fig. 2a, the levels of HDL-c and LDL-c tended to be higher in preterm UV, compared with term infants and not in umbilical artery (UA) (Fig. 2b). In maternal plasma, cholesterol level in the various classes of lipoproteins was not altered (Fig. 2c). The atherogenic index, plasma cholesterol/HDL-c ratio, was higher in UV plasma from preterm than from term infants (Fig. 3a). The cholesterol-associated lipoprotein concentration differed, with a non-significant increase in UV VLDL (+89%, p = 0.15), and a significant increase (+150%, p = 0.02) in UA VLDL in preterm, compared to term infants (Table 2). A strong trend toward higher levels was observed in HDL3 cholesterol in UA (+29%, p = 0.06) but not in UV. We found a trend towards lower TG in UV HDL2 (−50.5%, p = 0.08) in preterm compared to full-term infants (Table 2).
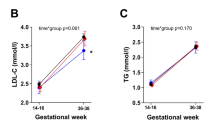
Plasma cholesterol (a) and triglycerides (TGs) (b) in maternal and fetal circulation in preterm infants with VLBW compared to term infants. Gray = preterm, Black = term, M-T = mother with term baby, M-PT = mother with preterm baby, UV-T = umbilical vein at term delivery, UV-PT = umbilical vein at preterm delivery, UA-T = umbilical artery at term delivery, UA-PT = umbilical artery at preterm delivery. Mean ± SD (n = 8 term, n = 7 preterm infants), *p < 0.05.
a Plasma cholesterol concentration in lipoprotein fractions in umbilical venous plasma in VLBW preterm compared to term infants, b in umbilical arterial plasma in VLBW preterm compared to term infants, and c in representative maternal plasma. In blue, UV-term = umbilical venous plasma at full term, UA-term = umbilical arterial plasma, MV-term = maternal venous plasma at full term; in red, UV-preterm = umbilical venous plasma, UA-preterm = umbilical arterial plasma, MV-preterm = maternal venous plasma in-preterm, Mean ± SD (n = 8 term, n = 7 preterm infants); VLDL very-low-density lipoprotein, IDL intermediate-density lipoprotein, LDL low-density lipoprotein, HDL high-density lipoprotein.
Plasma cholesterol/HDL-c, an atherogenic index, in preterm and term infants (a). Apolipoprotein A-I (ApoA-I) profiles in HDL2 in mother and fetal circulation in neonates born prematurely and at term (b). Gray = preterm, black = term, M-T = mother with term baby, M-PT = mother with preterm baby, UV-T = umbilical vein at term, UV-PT = umbilical vein at preterm delivery, UA-T = umbilical artery at term, UA-PT = umbilical artery at preterm delivery. Mean ± SD (n = 8 term, n = 7 preterm infants), *p < 0.05.
Prematurity was associated with decreased ApoA-I in fetal HDL2
We quantified apolipoproteins in several lipoproteins in maternal venous and UV and UA plasma (Table 3). Only ApoA-I, the major apolipoprotein of HDL, was significantly decreased in UV HDL2 in infants born prematurely (p = 0.042) but not in the circulation of their mothers (Fig. 3b).
Prematurity was associated with a profound change in fetal lipidome
From the untargeted lipidomic analysis, 33 lipid metabolites displayed changes in one (or more) of the 3 analyzed compartments (maternal blood, UV, UA). Interestingly, only 3 metabolites differed in mothers who delivered a preterm baby compared to mothers who delivered at full term, PC (36:2), PC (38:5), and hydroxyeicosatetraenoic acid (HETE) (Table 4) but were not significant after correction. However, HETE and eicosapentaenoic acid (EPA, 20:5n-3) were strongly correlated with each other in maternal blood (r = 0.9795, p < 0.0001). We also found a strong correlation between HETE in maternal plasma and cholesterol concentration in UV plasma (r = 0.6824, p < 0.005). There was a trend toward an inverse correlation between HETE and GA and BW (r = −0.4510, p = 0.091 and r = −0.4676, p = 0.079).
We identified many other alterations in umbilical plasma lipids in VLBW preterm, compared to term group. In both umbilical cord vessels (UV and UA), PC containing saturated fatty acids (SFAs) in both fatty acyl positions (sn1 and sn2) PC (32:0) and PC species with one unsaturation, such as PC (32:1) and PC (34:1), were markedly higher in VLBW preterm compared to term infants (Table 4). PC (32:0) and PC (34:1) were positively correlated with cholesterol level (r = 0.7716, p = 0.0008; r = 0.6301, p = 0.0118) in UV. Two PC species containing LCPUFA, PC (38:3) and PC (38:6), were deeply decreased in both umbilical vessels in preterm compared to term neonates. PC (38:6) was positively correlated with GA (r = 0.7545, p = 0.0012) and BW (r = 0.7662, p = 0.0009). Sphingolipids, including ceramide, Cer (m18:1/24:1), and most SM, SM (d18:1/16:0), SM (d18:1/16:1), SM (d18:1/18:0), SM (d18:1/18:1), and SM (d18:1/24:1), were increased in UV in preterm babies (Table 4). SM (d18:1/16:0) was increased in UA plasma and SM (d18:1/18:0) displayed a trend toward an increase in arterial plasma in VLBW neonates. Strong correlations were measured between SM (d18:1/16:0), SM (d18:1/16:1), SM (d18:1/18:0), SM (d18:1/18:1), and cholesterol level (r = 0.7239, p = 0.0022; r = 0.7264, p = 0.0023; r = 0.5633, p = 0.0288; r = 0.5633, p = 0.005) in UV plasma.
Only one species of TGs, TG (56:6), was significantly decreased in UV in preterm neonates, and TG (52:5) tended to decrease in UV (Table 4).
Among the unesterified fatty acids, palmitic acid (16:0, SFA) as well as palmitoleic acid (16:1n-7, monounsaturated fatty acid [MUFA]) tended to be lower in UV plasma of preterm infants (Table 4). Oleic acid (18:1n-9, MUFA) and linoleic acid (18:2n-6), an essential fatty acid (EFA) in the n-6 LCPUFA pathway, were also decreased by prematurity in fetal circulation in spite of the unchanged level in maternal circulation. We did not find any alteration in cholesteryl ester species in association with prematurity.
Discussion
This is the first study that characterize lipids and apolipoproteins carried in lipoproteins as well as lipidome in the two blood vessels of the umbilical cord in very preterm neonates. Observed results confirm that lipid profile is altered at the time of birth in preterm infants, compared with full-term infants. In addition, lipidomics data provide new insight into lipid-associated lipoprotein and lipidome profile in maternal–fetal unit in preterm births with VLBW. We particularly observed higher cholesterol in UV, which was driven by a trend toward enhanced cholesterol in VLDL, and higher VLDL-cholesterol in UA. ApoA-I concentration was decreased in HDL2 in UV. We also measured marked changes of fatty acid composition in phospholipid, particularly in SM and PC species, in preterm compared to full-term infants.
Maternal plasma cholesterol concentrations are known to increase considerably at late pregnancy (30–50% increase), especially in LDL,26 to meet the rising demand of the growing fetus, which triples its weight in the third trimester. We measured higher levels of cholesterol in UV in VLBW preterm compared to term infants, which is consistent with previous studies in preterm neonates.27,28 We also found similar plasma cholesterol in women who delivered prior to 32 weeks of gestation as in women who delivered at full term, suggesting that women delivering preterm presumably had abnormal elevated cholesterol levels early in pregnancy.29 The high cholesterol concentration in fetal circulation is thought to reflect a surge in cholesterol synthesis by the fast growing fetal liver between 20 and 32 weeks, which would exceed cholesterol utilization by fetal adrenal.30 In the current study, the higher cholesterol concentration in preterm fetal circulation may be due to the combination of (1) higher maternal supply and placental transfer, (2) excess cholesterol synthesis by the fetal liver, and (3) impaired fetal cholesterol utilization, e.g., for bile acid synthesis. Contrary to previous studies in preterm neonates, we assessed cholesterol levels separately in UV and UA plasma. Similar to UV, a trend toward higher cholesterol levels was measured in UA in VLBW preterm infants, suggesting enhanced return of fetal circulating cholesterol excess to the mother. This mechanism may reflect a metabolic adaptation to protect the fetus against accumulation of cholesterol.
The profile of cholesterol-associated lipoproteins is quite different between fetuses and mothers. As is already known, and regardless of GA, we confirmed that the major plasma lipoprotein in fetuses is HDL, rather than LDL as in adults.31,32 The difference in cholesterol profile, particularly in associated VLDL-c and LDL-c, in umbilical cord compared to maternal plasma, likely reflects immature hepatic function, resulting in a low synthesis rate of lipoproteins in fetal life. Interestingly, using native gel electrophoresis for HDL migration, Sreckovic et al. observed that fetal HDL migrated more slowly than maternal HDL.33 Our data show a shift in HDL fraction towards HDL3, in preterm plasma fetuses which confirms the predominance of smaller HDL in preterm compared to term neonates.
Increased plasma cholesterol of VLBW preterm neonates seems to be driven by a predominant increase in VLDL-c in both UV and UA, with no change in LDL and HDL. These results are in agreement with a study in late preterm infants,14 which showed higher VLDL-c in UV. Maturation of HDL is likely reduced in VLBW preterm compared to term infants.
We found that TG concentration remained similar in both umbilical cord vessels (UV and UA), and even in VLDL, in preterm compared to term infants, while higher TG level were reported in preterm neonates, especially in VLDL.7 This discrepancy in TG lipoprotein profile likely depends on GA. In healthy pregnancy, cord blood VLDL-TG concentrations significantly rise between 32 and 34 weeks of gestation.8 In our study, preterm babies were born prior to 32 weeks, which may explain the different TG results. Although TG concentration was unaltered in UV plasma in preterm, we found a trend toward a decrease of TG in large HDL2 and a decrease of specific TG species in UV plasma in infants born prior to 32 weeks of gestation.
Lipid and apolipoprotein composition is critical in maintaining HDL metabolism and function. After a rise between 21 and 34 weeks of gestation, the concentrations of the fetal ApoA-I associated with HDL remain stable until delivery, pointing out marked changes in fetal HDL composition between early and late pregnancy.34 Similar increase was observed between 28 and 32 weeks of gestation in fetal circulation of preterm babies.35 We found a lower ApoA-I concentration in fetal HDL2 in UV in VLBW neonates born prior to 32 weeks of gestation compared to term babies. In adults, reduced circulating levels of ApoA-I are associated with increased risk of atherosclerotic cardiovascular disease36 and type 2 diabetes.37 ApoA-I is a powerful activator of lecithin-cholesterol acyltransferase (LCAT) enzyme, which catalyzes the esterification of cholesterol activity thus leading the maturation of discoid pre-β-HDL into spherical α-HDL and further promotes cholesterol efflux from atherosclerotic plaques.38 LCAT enzyme activity is known to be low in preterm infants due to immaturity.39
LCAT activity also depends on the fluidity of phospholipid species, including PC and SM, that differed in fatty acyl chain length and degree of unsaturation. We found that PC-containing SFA and MUFA fatty acyl chains (PC (32:0), PC (32:1) and PC (32:1)) likely composed of 16 or 18 carbons, as well as SM containing 16:0, 18:0 and 16:1 and 18:1 increased in both umbilical cord vessels (UV and UA) of preterm compared to term neonates, altering fluidity parameters. Moreover, these PC and SM species were strongly correlated with cholesterol in UV, suggesting that these lipids may regulate cholesterol levels in fetal circulation. Upregulation of SM species was recently demonstrated in chorionic arteries of preeclamptic placentas with premature birth.40 In addition, SM content of HDL particles has been shown to inhibit LCAT enzyme activity,41,42 pointing to atherogenic properties of SM. Taken together, we speculate that lower ApoA-I in HDL2 and increased specific SM and PC species with alterations in their fatty acid content lead to a lower LCAT enzyme activity, resulting in cholesterol accumulation in fetal circulation of infants born prematurely exposing them to a higher cardiovascular risk as shown by increased atherogenic index.
While SFA- and MUFA-containing PC were increased in preterm neonates, we found decreased unesterified SFA (16:0), and MUFA (16:1n-7, 18:1n-9) levels in UV in preterm compared to term infants, whereas no change in these free FA contents was found in maternal circulation. Besides, the content of these unesterified FAs in UV correlated positively with BW suggesting either a transfer defect through the placenta or a lower fetal demand in the early third trimester of pregnancy. Level of linoleic acid (18:2n-6), an EFA, was also reduced in both umbilical cord vessels in VLBW preterm neonates and its level in UV was strongly correlated with GA and BW, confirming that EFA transfer and status in fetal circulation depends on GA.12,43
Impaired delivery of FAs and especially, LCPUFA such as docosahexaenoic acid (DHA), to the fetus, is believed to be deleterious for fetal brain development,5,44 in particular during the last trimester of pregnancy, a crucial period for brain growth.45,46 We found that unesterified DHA was unchanged in preterm neonate circulation, whereas PC (38:6) likely composed of DHA (22:6n-3) in sn2 and 16:0 in sn1 position was decreased in VLBW preterm compared to term neonates and was strongly correlated with GA and BW. These results corroborate studies showing decreased DHA in serum phospholipids in preterm neonates13 and those displaying a strong correlation between percentage of DHA in PC fraction in cord plasma of preterm neonates and GA.47 PC is the primary carrier in circulation for driving LCPUFA, and particularly DHA to several organ systems, such as placenta48,49 and brain. Decreased PC-containing DHA in premature infants may lead to reduced DHA uptake into the brain as previously reported50 and contribute to impaired neurodevelopment.
Interestingly, as was the case for DHA, no change was found in UV unesterified arachidonic acid (ARA, 20:4n-6), an n-6 LCPUFA also involved in fetal growth, suggesting that the placenta prioritizes the transfer of these LCPUFAs rather than other fatty acids, such as SFA and MUFA (C16:0 and C18:1 n-9), which were decreased in preterm births. However, ARA content tended to be enhanced in VLBW preterm umbilical artery compared to term, and much higher than preterm UV, suggesting that ARA was synthesized by fetus and exported to the placenta, presumably to protect the fetus from accumulation of ARA or its pro-inflammatory eicosanoid end products. Biosynthesis of LCPUFA from EFAs largely depends on the activity of specific desaturases and elongases, which is detectable as early as 26 weeks of gestation.51 Delta 6 desaturase was found to be particularly high at birth in preterm neonates52 and may explain the high ARA level in umbilical artery of VLBW neonates.
HETE showed a trend toward an increase and was strongly correlated with EPA (20:5n-3, n-3LCPUFA) level in maternal plasma of women who delivered prematurely, indicating that EPA likely is the main precursor of HETE. Increased EPA content into cell membranes results in the synthesis of alternative eicosanoids, which are less inflammatory than those produced from ARA.53 A recent study on predictive lipid biomarkers reported that 15-HETE and 12-HETE were associated with increased odds of preterm birth among many other lipid biomarkers in maternal circulation.54 Whereas we were not able to determine the exact configuration of HETE in our study, HETE level in maternal plasma tended to be inversely correlated with GA, likely pointing out a marker of prematurity. HETE in maternal circulation was also strongly correlated with cholesterol level in UV. Although it has been reported that HETE and in particular 15-HETE is involved in cardiovascular disease,55,56 whether increased HETE level in maternal circulation that impacts cholesterol concentration in fetal circulation associated with an enhanced placental transfer of cholesterol to the fetus in women delivered prematurely, is unknown and will require further investigations.
Relevance
We believe our findings suggest that further studies should be designed to address two issues.
First, as this was a pilot study, the alterations observed in lipidome and lipoprotein profile in the current study should be confirmed in a larger population of very preterm infants at birth. Second, long-term cohort studies should be designed to assess (a) neurodevelopment at 5 years of age, e.g., using ages and stage questionnaires as performed in our earlier studies57 and (b) early markers of metabolic outcome such as Homeostatic Model Assessment for Insulin Resistance index at 7–10 years of age38; such studies would be needed to ascertain whether altered lipidome and lipid transfer correlate with outcome and could be used as early biomarkers of long-term outcome. If so, specific preventive nutritional strategies could be targeted to mitigate the risk of poor metabolic or neurologic outcome in those who are most at risk among the population of preterm infants.
Strengths and limitations
Small sample size clearly is a limitation of our study. The lack of placental tissue analysis to detect the alterations of specific lipid transporters or enzyme activity involved in lipid metabolism and the small volume of cord blood available that prevented measuring LCAT enzyme activity in fetal plasma are other limitations. Despite such limitations, the strength of our study stems from the lipidomic analysis in plasma and the lipid and apolipoprotein contents in every lipoprotein in paired mother–offspring dyads. The simultaneous assessment in both umbilical blood vessels is another unique strength, as it sheds light on the potential contribution of the placenta to the altered composition of lipid and lipoprotein profiles in preterm, compared to term neonates.
Conclusion
To the best of our knowledge, this pilot study is the first to describe the alterations in plasma lipidome, cholesterol, and TG-associated lipoprotein and apolipoprotein profiles in a mother–fetal unit in VLBW preterm, compared to term birth. Our findings reveal lower ApoA-I concentration in large HDL2, increased PC species containing SFA and MUFA, and enhanced SM and cholesterol levels in UV plasma in VLBW preterm neonates, which may, in turn, lead to atherosclerotic lesions later in life. We also found decreased PC-containing LCPUFA such as PC (38:6), which suggests a reduction in DHA availability for brain development in VLBW preterm infants. Our findings suggest that postnatal nutritional strategies should be developed to mitigate the lack of specific lipid nutrients particularly targeting the fatty acid composition of PC. Nevertheless, the findings from this pilot study clearly warrant confirmation in a larger cohort to establish the key role of placenta in alterations of lipid nutrient transfer to the preterm infant.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Rush, R. W. et al. Contribution of preterm delivery to perinatal mortality. Br. Med. J. 2, 965–968 (1976).
Martin, J. A., Hamilton, B. E., Osterman, M. J. K., Driscoll, A. K. & Drake, P. Births: final data for 2016. Natl Vital Stat. Rep. 67, 1–55 (2018).
Robinson, D. T. & Martin, C. R. Fatty acid requirements for the preterm infant. Semin. Fetal Neonatal Med. 22, 8–14 (2017).
Murray, E. et al. Differential effect of intrauterine growth restriction on childhood neurodevelopment: a systematic review. BJOG 122, 1062–1072 (2015).
Contu, L. & Hawkes, C. A. A review of the impact of maternal obesity on the cognitive function and mental health of the offspring. Int. J. Mol. Sci. 18, 1093 (2017).
Godfrey, K. M. & Barker, D. J. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 71, 1344S–1352S (2000).
Nagano, N. et al. Early postnatal changes of lipoprotein subclass profile in late preterm infants. Clin. Chim. Acta 413, 109–112 (2012).
Ryuta Yonezawa, T. O. et al. Very low-density lipoprotein in the cord blood of preterm neonates. Metabolism 58, 704–707 (2009).
Tea, I. et al. 1H-NMR-based metabolic profiling of maternal and umbilical cord blood indicates altered materno-foetal nutrient exchange in preterm infants. PLoS ONE 7, e29947 (2012).
Pardo, I. M., Geloneze, B., Tambascia, M. A. & Barros-Filho, A. A. Atherogenic lipid profile of Brazilian near-term newborns. Braz. J. Med Biol. Res. 38, 755–760 (2005).
Posod, A. et al. Apolipoprotein profiles in very preterm and term-born preschool children. J. Am. Heart Assoc. 8, e011199 (2019).
Foreman-van Drongelen, M., M., al, M. D., van Houwelingen, A. C., Blanco, C. E. & Hornstra, G. Comparison between the essential fatty acid status of preterm and full-term infants, measured in umbilical vessel walls. Early Hum. Dev. 42, 241–251 (1995).
Arsic, A. et al. Different fatty acid composition of serum phospholipids of small and appropriate for gestational age preterm infants and of milk from their mothers. Hippokratia 16, 230–235 (2012).
Katragadda, T., Mahabala, R. S., Shetty, S. & Baliga, S. Comparison of cord blood lipid profile in preterm small for gestational age and appropriate for gestational age newborns. J. Clin. Diagn. Res. 11, SC05–SC07 (2017).
Kuster, A. et al. Cord blood glutathione depletion in preterm infants: correlation with maternal cysteine depletion. PLoS ONE 6, e27626 (2011).
Barfield, W. D. Public health implications of very preterm birth. Clin. Perinatol. 45, 565–577 (2018).
Chétiveaux, M. et al. The differential apoA-I enrichment of preb1 and aHDL is detectable by gel filtration separation. J. Lipid Res. 43, 1986–1993 (2002).
Croyal, M. et al. Multiplexed peptide analysis for kinetic measurements of major human apolipoproteins by LC/MS/MS. J. Lipid Res. 57, 509–515 (2016).
Blanchard, V. et al. A high-throughput mass spectrometry-based assay for large-scale profiling of circulating human apolipoproteins. J. Lipid Res. 61, 1128–1139 (2020).
Ferchaud-Roucher, V. et al. Plasma lipidome analysis by liquid chromatography-high resolution mass spectrometry and ion mobility of hypertriglyceridemic patients on extended-release nicotinic acid: a pilot study. Cardiovasc. Drugs Ther. 31, 269–279 (2017).
Croyal, M. et al. Fenofibrate decreases plasma ceramide in type 2 diabetes patients:a novel marker of CVD? Diabetes Metab. 44, 143–149 (2018).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959).
Gallart-Ayala, H. et al. Versatile lipid profiling by liquid chromatography-high resolution mass spectrometry using all ion fragmentation and polarity switching. Preliminary application for serum samples phenotyping related to canine mammary cancer. Anal. Chim. Acta 796, 75–83 (2013).
Storey, J. D. A direct approach to false discovery rates. J. R. Stat. Soc. Ser. B Stat. Methodol. 64, 479–498 (2002).
Ouguerram, K. et al. Apolipoprotein B100 metabolism in autosomal-dominant hypercholesterolemia related to mutations in PCSK9. Arterioscler Thromb. Vasc. Biol. 24, 1448–1453 (2004).
Chiang, A. N. et al. Alterations of serum lipid levels and their biological relevances during and after pregnancy. Life Sci. 56, 2367–2375 (1995).
Diaz, M. et al. Cord blood lipoprotein-cholesterol: relationship birth weight and gestational age of newborns. Metabolism 38, 435–438 (1989).
Pecks, U. et al. Cholesterol acceptor capacity is preserved by different mechanisms in preterm and term fetuses. Biochim. Biophys. Acta 1841, 251–258 (2014).
Napoli, C. et al. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J. Clin. Investig. 100, 2680–2690 (1997).
Johnson, H. J. Jr., Simpson, E. R., Carr, B. R., MacDonald, P. C. & Parker, R. C. Jr. The levels of plasma cholesterol in the human fetus throughout gestation. Pediatr. Res. 16, 682–683 (1982).
Dolphin, P. J., Breckenridge, W. C., Dolphin, M. A. & Tan, M. H. The lipoproteins of human umbilical cord blood apolipoprotein and lipid levels. Atherosclerosis 51, 109–122 (1984).
Lane, D. M. & McConathy, W. J. Changes in the serum lipids and apolipoproteins in the first four weeks of life. Pediatr. Res. 20, 332–337 (1986).
Sreckovic, I. et al. Distinct composition of human fetal HDL attenuates its anti-oxidative capacity. Biochim. Biophys. Acta 1831, 737–746 (2013).
Parker, C. R. Jr. et al. Apolipoprotein A-1 in umbilical cord blood of newborn infants: relation to gestational age and high-density lipoprotein cholesterol. Pediatr. Res. 23, 348–351 (1988).
Hellgren, G., Engstrom, E., Smith, L. E., Lofqvist, C. & Hellstrom, A. Effect of preterm birth on postnatal apolipoprotein and adipocytokine profiles. Neonatology 108, 16–22 (2015).
McQueen, M. J. et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet 372, 224–233 (2008).
Feng, X., Gao, X., Yao, Z. & Xu, Y. Low apoA-I is associated with insulin resistance in patients with impaired glucose tolerance: a cross-sectional study. Lipids Health Dis. 16, 69 (2017).
Hofman, P. L. et al. Premature birth and later insulin resistance. N. Engl. J. Med. 351, 2179–2186 (2004).
Hamosh, M. Lipid metabolism in premature infants. Biol. Neonate 52(Suppl 1), 50–64 (1987).
Del Gaudio, I., Sasset, L., Lorenzo, A. D. & Wadsack, C. Sphingolipid signature of human feto-placental vasculature in preeclampsia. Int. J. Mol. Sci. 21, 1019 (2020).
Bolin, D. J. & Jonas, A. Sphingomyelin inhibits the lecithin-cholesterol acyltransferase reaction with reconstituted high density lipoproteins by decreasing enzyme binding. J. Biol. Chem. 271, 19152–19158 (1996).
Subbaiah, P. V., Horvath, P. & Achar, S. B. Regulation of the activity and fatty acid specificity of lecithin-cholesterol acyltransferase by sphingomyelin and its metabolites, ceramide and ceramide phosphate. Biochemistry 45, 5029–5038 (2006).
Dutta-Roy, A. K. Transport mechanisms for long-chain polyunsaturated fatty acids in the human placenta. Am. J. Clin. Nutr. 71, 315S–322S (2000).
Rogers, L. K., Valentine, C. J. & Keim, S. A. DHA supplementation: current implications in pregnancy and childhood. Pharmacol. Res. 70, 13–19 (2013).
Huppi, P. S. et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann. Neurol. 43, 224–235 (1998).
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8, 110–124 (2009).
Bernhard, W. et al. Transport of long-chain polyunsaturated fatty acids in preterm infant plasma is dominated by phosphatidylcholine. Eur. J. Nutr. 57, 2105–2112 (2018).
Ferchaud-Roucher, V. et al. A potential role for lysophosphatidylcholine in the delivery of long chain polyunsaturated fatty acids to the fetal circulation. Biochim Biophys. Acta Mol. Cell Biol. Lipids 1864, 394–402 (2019).
Powell, T. L. et al. Sex-specific responses in placental fatty acid oxidation, esterification and transfer capacity to maternal obesity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1866, 158861 (2021).
Martinez, M. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 120, S129–S138 (1992).
Clandinin, M. T., Chappell, J. E., Heim, T., Swyer, P. R. & Chance, G. W. Fatty acid utilization in perinatal de novo synthesis of tissues. Early Hum. Dev. 5, 355–366 (1981).
Nagano, N. et al. Delta-6 desaturase activity during the first year of life in preterm infants. Prostaglandins Leukotrienes Essent. Fat. Acids 115, 8–11 (2016).
Calder, P. C. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol. Nutr. Food Res. 52, 885–897 (2008).
Aung, M. T. et al. Prediction and associations of preterm birth and its subtypes with eicosanoid enzymatic pathways and inflammatory markers. Sci. Rep. 9, 17049 (2019).
Henriksson, P., Hamberg, M. & Diczfalusy, U. Formation of 15-HETE as a major hydroxyeicosatetraenoic acid in the atherosclerotic vessel wall. Biochim. Biophys. Acta 834, 272–274 (1985).
Lundqvist, A. et al. The arachidonate 15-lipoxygenase enzyme product 15-HETE is present in heart tissue from patients with ischemic heart disease and enhances clot formation. PLoS ONE 11, e0161629 (2016).
Boquien, C. Y. et al. Breast milk protein content at week 3 after birth and neurodevelopmental outcome in preterm infants fed fortified breast milk. Eur. J. Nutr. 60, 3959–3969 (2021).
Acknowledgements
The authors are grateful to the staff of the Mère-Enfant Clinical Investigation Centre of University Hospital in Nantes for their invaluable help in the management of the blood collection. The authors thank Caroline Mallier for her technical assistance. Lipidomic analysis and apolipoprotein quantification were performed in the Mass Spectrometry Core Facility of CRNH Ouest, Biogenouest Corsaire and SFR F. Bonamy UMS 016 at the University of Nantes.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
A.K. designed the study protocol and provided the human samples. M.C. quantified apolipoprotein in the lipoprotein fractions. D.D. and K.O. supervised the experiments. V.F.-R. conducted the lipidomic analysis, analyzed the results, supervised the experiments, and wrote the manuscript. T.M. supervised the statistical analyses. All authors discussed the results, edited the manuscript, and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
A written informed consent was obtained from every woman included in this clinical pilot study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Küster, A., Croyal, M., Moyon, T. et al. Characterization of lipoproteins and associated lipidome in very preterm infants: a pilot study. Pediatr Res 93, 938–947 (2023). https://doi.org/10.1038/s41390-022-02159-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02159-9