Abstract
Background
To examine the impact of PRBC transfusion on pulmonary vascular resistance (PVR), systemic vascular resistance and myocardial function using echocardiography and cerebral and splanchnic tissue oxygenation using near-infrared spectroscopy (NIRS) in premature babies with and without a PDA.
Methods
A prospective observational study of premature infants born <1500 g in receipt of PRBC transfusions beyond 10 days of age. Echocardiography and NIRS monitoring were performed at baseline, during the transfusion and 24 h after transfusion.
Results
Thirty infants with a median gestation of 26.4 [24.8–28.0] weeks were enrolled. Ten infants had a PDA. Following transfusion, a significant decrease in PVR markers occurred in all infants. Right ventricular (RV) function increased following transfusion in the PDA closed group only. Cerebral oxygen saturation increased following transfusion in all infants. Babies in the PDA open group had significantly lower splanchnic oxygen saturations at baseline compared to the PDA closed group which persisted over the study period and were unaltered by transfusion.
Conclusions
PRBC transfusion lowers PVR irrespective of PDA status. Those with a PDA demonstrated a lack of improvement in RV function and splanchnic oxygenation highlighting the impact a PDA has on the neonatal circulation.
Impact
-
The presence or absence of the PDA imposes differential effects on splanchnic oxygenation during red blood cell (PRBC) transfusion in the premature population.
-
This is the first study to assess the impact of the PDA on splanchnic oxygenation via near-infrared spectroscopy (NIRS) during red blood cell transfusion in premature neonates.
-
New insights have been found into the impact of PRBC transfusion on pulmonary vascular resistance, right ventricular function, cerebral and splanchnic oxygenation in the presence and absence of a PDA and emphasises the ongoing impact of ductal patency on gut oxygenation
Similar content being viewed by others
Introduction
Neonatal anaemia is common in the premature population with up to 40% of low birth weight infants and up to 90% of extremely low birth weight infants receiving packed red blood cell transfusions (PRBCs) in the weeks following delivery.1,2 Neonatal anaemia may occur secondary to acute blood loss, decreased red blood cell production, increased erythrocyte destruction and phlebotomy.3 However, the potential impact of a neonatal PRBC transfusion on neonatal haemodynamics including end-organ blood flow and myocardial performance has not been fully elucidated. In addition, the presence of a patent ductus arteriosus (PDA) may further contribute to potential haemodynamic changes consequent to a PRBC transfusion. There is a need to better characterise the effects of PRBC transfusion on neonatal haemodynamics and myocardial performance, both in the presence and absence of a PDA.
There is conflicting evidence regarding the specific impact a PRBC transfusion may impose on pulmonary vascular resistance (PVR), systemic vascular resistance (SVR) and myocardial function in the neonatal population.4,5,6 There is also a dearth of information regarding how such haemodynamic effects may be further modulated in the presence or absence of a PDA. To date, no study has assessed the impact of neonatal PRBC transfusion in the presence of a PDA on the haemodynamic status of premature infants, and whether there are different physiological phenotypes between infants with and without a PDA following a PRBC transfusion.7 We hypothesise that a PRBC transfusion will impose divergent haemodynamic effects in the presence and absence of a PDA. Therefore, the primary aim of this study was to examine the haemodynamic impact of PRBC transfusion on PVR, SVR and myocardial function measured using echocardiography, in addition to cerebral and splanchnic tissue oxygenation in premature infants with and without a PDA.
Methods
This was a prospective observational study carried out at the Rotunda Hospital, Dublin, Ireland between November 2019 and February 2021. All premature infants <1500 g in receipt of red blood cell transfusions beyond the first 10 days of age were eligible for inclusion in this study. This was done to ensure the mitigation of any confounding effects resulting from haemodynamic instability or the transitional circulation that may occur during the perinatal period. We wanted to assess the impact of a PRBC transfusion in infants with isolated anaemia of prematurity. Infants were excluded if there was a lack of parental consent, a congenital anomaly including congenital heart disease (other than a PDA), or if there was a high likelihood of death over the first week of age. Following recruitment antenatal, birth and neonatal characteristics were collected including; gestation, birthweight, sex, age at transfusion, and pre- and post-transfusion haemoglobin levels. We collected the following clinical outcomes: intraventricular haemorrhage occurring at any stage throughout the hospital stay, the use of inotropes, culture-proven sepsis, necrotising enterocolitis, the use of postnatal steroids, chronic lung disease defined at the use of oxygen by 36 weeks postmenstrual age, retinopathy of prematurity, and death before discharge. Ethical approval for this study was approved by the Rotunda Hospital ethics committees and informed written consent was obtained from all parents prior to enrolment.
In our institution, infants are transfused if their haemoglobin is under 10 g/dL if invasively ventilated, under 8 g/dL if on non-invasive ventilation and under 8 g/dL if in room air. We do not use prophylactic indomethacin. PDA treatment is currently performed under the discretion of the attending neonatologist. This usually occurs is an infant who is ventilated beyond the first week of age following an echocardiography assessment demonstrating the presence of a PDA with evidence of pulmonary over circulation (left ventricular output >300 mL/kg/min, left atrial to aortic root ratio >2, mitral valve e:a wave ratio >1) and systemic hypoperfusion (absent or reversed diastolic flow in the descending aortic and coeliac arteries). We use ibuprofen (10 mg/kg, 5 mg/kg and 5 mg/kg 24 h apart) as a first-line treatment. A course is repeated if PDA closure is not demonstrated after the first course.
Echocardiography assessment
Echocardiography scans were performed at three time periods, pre, 18 h post and 24 h post PRBC transfusion. Those time points were selected to ensure investigator availability to conduct the scans. Non-urgent blood transfusions are usually carried out in the late afternoon in our unit and as such those timings were chosen to minimise out-of-hours echocardiography assessments. Evaluations were performed using the Vivid echocardiography system (GE Medical, Milwaukee) and a cardiology multi-frequency probe. No sedation was used during the scans. All scans were recorded on the machine’s internal hard drive and transferred to the EchoPAC archiving system (version 202) for offline measurements. Studies were performed using standard neonatal windows including apical, parasternal, subcostal, and high parasternal windows in accordance with published guidelines.8,9 The following measurements were be obtained, a detailed description of the methodology, feasibility and reliability is published elsewhere.10,11,12,13,14,15 Measures of pulmonary vascular resistance (PVR) including pulmonary artery acceleration time (PAAT), PAAT indexed to right ventricular ejection time (PAAT:RVET) and left ventricular eccentricity index (LV EI); a surrogate measure of SVR using LV end systolic wall stress (ESWS) was calculated using the following formula by Wilson et al. where SBP = systolic blood pressure; LVESD = left ventricular end systolic diameter; and LVPWs = left ventricular posterior wall thickness in systole16:
Myocardial function and PDA characteristics were further evaluated by left global longitudinal strain (LV GLS) and right ventricular free wall strain (RVFwS) using speckle tracking echocardiography (STE), PDA diameter and maximum systolic velocity across the duct. We also measured the velocity time index (VTI) of the brachiocephalic artery (BCA) and the coeliac artery in addition to descending aortic end diastolic velocity (EDF).
Near infrared spectroscopy (NIRS) monitoring
NIRS monitoring of regional cerebral and splanchnic mixed venous saturations (rSO2) was performed using the INVOS Somanetics System (Medtronic, MN) and oximetry neonatal sensors. For cerebral NIRS, the sensors were applied to the skull over the frontal lobes at the centre of the forehead at baseline, for the duration of the transfusion and the 24 h following the transfusion. The hypoxic threshold for cerebral saturation in this system is 63%. For splanchnic NIRS, the sensor was applied to the centre of the abdomen, below the umbilicus as per vendor instructions at baseline, for the duration of the transfusion and the 24 h following the transfusion. Regional tissue oxygenation (rSO2) is calculated by NIRS utilising the variability in absorption spectra of oxygenated and deoxygenated haemoglobin molecules.17 The NIRS probe emits light that is absorbed, scattered and reflected by the underlying tissue and detectors in the NIRS probe record the amount of reflected light; a measurement of the organ-specific ratio of oxygen supply and consumption.17 The organ-specific fractional tissue oxygen extraction (FTOE) was calculated by simultaneously measuring SpO2 and deriving FTOE using the following equation: FTOE = (SpO2 − rSO2)/SpO2. Peripheral oxygen saturations were measured from the lower limbs as per unit protocol in stable infants.
Statistical analysis
The cohort was divided into two groups: Infants with a PDA at the time of transfusion and those without a PDA. Continuous data were tested for normality using Shapiro–Wilk test and a histogram representation of data and summarised as means (standard deviation) or medians [interquartile range (IQR)] as appropriate. Categorical data were summarised as counts (%). Two-group analyses were conducted using Student’s t test, Mann–Whitney U test or Chi-square test as appropriate. Serial data were compared using one- or two-way analysis of variance with repeated measures. Correlations between continuous variables were conducted using Spearman’s correlation coefficient. We used SPSS version 25 to conduct the analyses. Statistical significance is achieved with a p value <0.05.
Results
Clinical parameters and baseline PDA characteristics
Thirty infants with a median [IQR] gestation and birth weight of 26.4 [24.8–28.0] weeks and 855 [659–1102] g, respectively, were prospectively enrolled in the study. Ten infants had a PDA at the time of the transfusion: they were of a lower gestation and birth weight, with a higher rate of invasive ventilation. None of the infants were lower than the 10th centile for weight at birth. Infants in the PDA group had a lower descending aortic EDF and a lower coeliac artery VTI, but no difference in BCA VTI was observed (Table 1). There were no other differences in the remainder of the demographics between infants with and without a PDA (Table 1). There were no differences in any of the recorded outcomes between the two groups (Table 2). There was no change in PDA diameter over the three echocardiograms: Baseline: 2.2 [1.7–2.7], 18 h: 2.1 [1.7–2.6], 24 h: 2.0 [1.7–2.9] mm (p = 0.64). There was a fall in the systolic velocity across the PDA over the three echocardiograms (2.4 ± 0.6 vs. 2.0 ± 1.0 vs. 2.0 ± 0.9 m/s, p = 0.03).
Echocardiography measurements
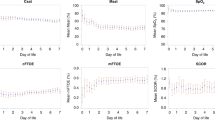
Following the PRBC transfusion, there was an increase in PAAT and in the PAAT:RVET ratio, and a corresponding fall in LV EI in the entire cohort (Fig. 1). There were no differences in PAAT, PAAT:RVET or LV EI between the two groups at any time point (data not shown, all p > 0.05). No changes were observed in LV ESWS following a PRBC transfusion in the cohort (330 [220–427] vs. 333 [265–385] vs. 328 [275–414] dynes/cm2, p = 0.54). There was no difference in BCA VTI between the two groups across the three time points. However, coeliac artery VTI was lower in the PDA group throughout the study period with significant differences occurring on the first and third scans, there were no changes in the coeliac artery VTI in either group over time (Fig. 2).
In the closed PDA group, RV function increased over the three echocardiograms following the transfusion (Fig. 2). There was no change in RV function in the PDA group throughout the study period (Fig. 2). There was no change in LV function following the transfusion with no differences noted between the groups at any time point (Fig. 2).
NIRS measurements
Infants in the PDA group had a higher rate of being below the hypoxic threshold at baseline (non-significant trend, Table 3); however, we did not observe a correlation between PDA diameter, BCA VTI or coeliac artery VTI with any of the NIRS measurements (all r < 0.3, all p > 0.05). Following the PRBC transfusion, there was an increase in cerebral oxygen saturations and a corresponding fall in cerebral FTOE in both groups with no differences noted between infants with and without a PDA over the three echocardiograms; only one infant in each group was below the hypoxic threshold at 24 h post transfusion (Fig. 3 and Table 3). Infants with a PDA had lower baseline splanchnic oxygen saturations and higher baseline splanchnic FTOE compared with infants without a PDA; those differences persisted throughout the study period (Fig. 3 and Table 3). There was no change in splanchnic oxygen saturations or splanchnic FTOE following a transfusion in the PDA group. In the Closed PDA group, splanchnic oxygen saturations increased, and splanchnic FTOE decreased following the transfusion (Fig. 3 and Table 3).
Discussion
This is the first study to prospectively evaluate premature infants with echocardiography and NIRS to demonstrate divergent effects of PRBC transfusion on splanchnic NIRS measurements in those with and without a PDA. A PRBC transfusion appears to have a PVR lowering effect irrespective of PDA status, however, infants with a PDA appear to have a suboptimal response to a PRBC transfusion evident in the splanchnic circulation.
Impact of PRBC transfusion on indices of PVR
Our data demonstrated that following PRBC transfusion there was a significant change in PAAT, PAAT:RVET and LV EI indicating a fall in PVR, which occurred independently of the PDA status. The relationship between PRBC transfusion and PVR is complex and has been investigated in a variety of animal and human studies.4 Oxygen delivery and arterial oxygen content are significantly increased following PRBC transfusion.18 However, rodent models have reported that polycythaemia with haematocrits between 50 and 60% contribute to the evolution of pulmonary hypertension through increased blood viscosity.4 Adult studies have suggested increased pulmonary artery pressure and pulmonary vascular resistance indices following PRBC transfusion through pulmonary vasoconstriction, with such increases occurring to a greater extent when patients are transfused with PRBCs stored over 21 days in comparison to PRBCs stored for <14 days.19 In the paediatric population, increasing haemoglobin concentration and haematocrit levels while maintaining a constant blood volume through isovolumic exchange transfusion in nine infants with a large left to right cardiac shunts was associated with a rise in systemic and pulmonary vascular resistances, a concomitant decrease in systemic and pulmonary blood flow and marked reduction in their left to right shunt.7 It is possible that the administration of PRBC transfusions to anaemic individuals aiming to normalise haematocrit levels will drop PVR through increased oxygen delivery to the pulmonary vascular tissue without a change in blood viscosity; however, after a critical point ongoing increases in haematocrit will induce rising PVR secondary to polycythaemia. Further research within the neonatal population will be required to further clarify this relationship.
Impact of PRBC transfusion on myocardial function
Previous studies have documented increased stroke volume and LV output in anaemic premature infants, which normalise to values comparable to non-anaemic premature infants following PRBC transfusion.20,21,22 Our results demonstrated no change in LV GLS or LV ESWS over the study period, a finding mirrored by Radicioni et al. who also reported no change in LV ESWS following PRBC transfusion in 32 anaemic premature babies.23 Utilising tissue Doppler imaging, Saleemi et al. documented an improvement in LV systolic and LV diastolic velocities on days 3–5 following PRBC transfusion compared to non-transfused controls.24 It is possible that our assessment of myocardial function at 24 h following PRBC transfusion was too early to detect any considerable alterations in LV function and that later evaluation on days 3–5 post transfusion similar may have yielded different results. Alkalay et al. postulated that a proportion of anaemic premature infants are in a clinically unrecognised high output cardiac state and that despite PRBC transfusions some echocardiographic measurements did not improve; potentially it takes some time for post-transfusion myocardial adaptation to occur.25
We observed a significant increase in RV free wall strain following PRBC transfusion in the no PDA group. This improvement in RV function is likely related to the observed drop in PVR indices and reduced RV afterload. There was no change in RV function in the PDA group throughout the study period. No demonstrable change in PDA calibre was observed following the transfusion. However, we saw a small fall in PDA systolic velocity by 24 h following a transfusion. This change is not in keeping with the lower PVR observed as an increase in the pressure gradient across the duct is expected to increase the velocity of blood flow. The small number of infants in this study precludes us from investigating this observation further.
Impact of PRBC transfusion on cerebral and splanchnic perfusion
Our results document an increase in cerebral oxygen saturation and a corresponding decrease in cerebral FTOE over the study period in both the open and closed PDA groups. Our findings are consistent with previous publications; Mintzer et al. reported a 10% increase in cerebral tissue oxygenation with a concomitant decrease in FTOE in ten neonates with a median gestational age of 26 weeks who received PRBC transfusion over the first week of age and demonstrated increased cerebral oxygenation saturations and decreased FTOE values following PRBC transfusion in a later study of 23 premature infants born <30 weeks gestation over 1 month of age.26,27 Those findings are also replicated in several other studies.28,29,30
Interestingly, our data revealed differential effects of PRBC transfusion on splanchnic NIRS measurements between premature infants with and without a PDA. Babies in the PDA open group have significantly lower splanchnic oxygen saturations and higher splanchnic FTOE values compared to the PDA closed group at baseline which persisted over the study period and were unaltered by PRBC transfusion. In contrast, the PDA closed group experienced a significant increase in splanchnic oxygen saturation and a decrease in splanchnic FTOE values following PRBC transfusion. The integration of echocardiography and NIRS assessments in this cohort may help explain the differential response between cerebral and splanchnic saturation. Echocardiography assessment of the BCA (a surrogate for cerebral perfusion) and the coeliac trunk (a surrogate for splanchnic perfusion) demonstrate a significant difference in flow in the coeliac trunk between infants with and without a PDA with infants with a PDA demonstrating lower coeliac VTI values; the coeliac trunk is a post ductal vessel and it, therefore, is more likely to be adversely affected by left to right PDA shunting. However, no such differences in flow exist in the BCA. This preductal vessel may be less affected by the left to right shunting of the PDA. Oxygen delivery (DO2) to tissues is dependent of blood oxygen content (CaO2) and cardiac output. Addressing only CaO2 by increasing haemoglobin concentration is therefore unlikely to improve DO2 in the setting of low systemic blood flow secondary to the PDA.
There is evidence however illustrating that a haemodynamically significant PDA can eventually affect the preductal cerebral circulation. Poon et al. examined cerebral NIRS measurements in 29 premature infants pre and post medical/surgical closure of hsPDA and reported a significant increase in cerebral oxygen saturations following PDA treatment with a concomitant decrease in FTOE values. In addition, this work found no correlation between NIRS values and PDA size.31 Several other studies have also demonstrated lower cerebral tissue oxygenation in the presence of a PDA, especially when persisting and failing medical treatment.32,33 This negative effect appears to be more pronounced in small for gestational age infants.34
The impact of PDA on the post ductal circulation has been previously demonstrated using NIRS. A recent study reported lower renal oxygen saturation in infants with a haemodynamically significant PDA (hsPDA) in comparison to infants with a non-hsPDA while cerebral oxygen saturations were equivalent between hsPDA and non-hsPDA groups.35 Such findings support the concept of cerebral autoregulation as a protective mechanism,36 and the contrasting effect a PDA has on pre- and post-ductal blood vessels. Ledo et al. also documented a significant relationship between a hsPDA and lower regional splanchnic oxygenation.37
Previous studies have also assessed the impact of PRBC transfusion on splanchnic NIRS; however, none have done so while taking the presence of a PDA into consideration. Aktas et al. documented a significant increase in abdominal oxygenation saturation measurements following PRBC transfusion in their study of 35 preterm infants born < 33 weeks gestation and Banerjee et al. also reported that while mean superior mesenteric artery peak systolic velocity and diastolic velocities were unaltered by neonatal PRBC transfusion there was a significant increase in splanchnic tissue oxygenation and a significant decrease in splanchnic FTOE in extremely preterm infants post transfusion.38,39 Significantly greater variability in splanchnic tissue oxygenation saturation values compared to cerebral tissue oxygen saturation values during neonatal PRBC transfusion have also been reported.40 Marin et al. reported that four very low birth weight (VLBW) infants who developed transfusion-related necrotising enterocolitis (TFNEC) demonstrated greater variation in mesenteric oxygenation values during PRBC transfusions in comparison to four VLBW infants who did not develop TFNEC.41 It is postulated that a rapid increase in abdominal tissue oxygenation following PRBC transfusion may cause ischaemia–reperfusion injury through oxidative stress and potentially trigger NEC.38,40,42 However, the debate surrounding the association between PRBC transfusion and NEC is very much ongoing with a 2018 systematic review and meta-analysis finding a statistically significant reduction in the odds of NEC in infants exposed to PRBC transfusion (odds ratio = 0.55, 95% confidence interval = 0.31 − 0.98) and postulated that PRBC transfusion may in fact be protective against the development of NEC.43
The limitations of this work are the small numbers of infants included in this pilot study. Due to the low incidence of serious adverse outcomes in our cohort, we were precluded from assessing the relationship between low splanchnic saturations and the development of NEC. We did not assess preductal arterial saturation in this cohort and as such the calculation of cerebral FTOE may not be accurate. We would have preferred to obtain an echocardiogram 4–6 h after the initiation of a blood transfusion to match the NIRS assessment time points. This however was not feasible as this time point usually occurred after hours. We did not observe an improvement in myocardial function following PRBC transfusion in contrast to earlier studies, possibly due to the timing of the echocardiogram being performed too early post transfusion. Potential future directions for this work include adequately powered studies investigating the haemodynamic impact of the PDA in the context of PRBC transfusion on PVR, myocardial function, cerebral and gut perfusion and oxygenation and associations with neonatal morbidity including IVH and NEC.
In conclusion, this work has gleaned new insights into the impact of PRBC transfusion on PVR, RV function, cerebral and splanchnic oxygenation in the presence and absence of a PDA. Our data emphasise the ongoing impact of ductal patency on gut oxygenation and further studies are required to fully elucidate the potential clinical impact of this observation on important neonatal outcomes.
References
Howarth, C., Banerjee, J. & Aladangady, N. Red blood cell transfusion in preterm infants: current evidence and controversies. Neonatology 114, 7–16 (2018).
Villeneuve, A., Arsenault, V., Lacroix, J. & Tucci, M. Neonatal red blood cell transfusion. Vox Sang. 116, 366–378 (2021).
Aher, S., Malwatkar, K. & Kadam, S. Neonatal anemia. Semin. Fetal Neonatal Med. 13, 239–247 (2008).
Barer, G. R., Bee, D. & Wach, R. A. Contribution of polycythaemia to pulmonary hypertension in simulated high altitude in rats. J. Physiol. 336, 27–38 (1983).
Loer, S. A., Scheeren, T. W. & Peters, J. Interaction between haematocrit and pulmonary blood volume on pulmonary vascular flow resistance and pressure flow relationships. Intensive Care Med. 23, 1082–1088 (1997).
Nihill, M. R., McNamara, D. G. & Vick, R. L. The effects of increased blood viscosity on pulmonary vascular resistance. Am. Heart J. 92, 65–72 (1976).
Lister, G., Hellenbrand, W. E., Kleinman, C. S. & Talner, N. S. Physiologic effects of increasing hemoglobin concentration in left-to-right shunting in infants with ventricular septal defects. N. Engl. J. Med. 306, 502–506 (1982).
de Boode, W. P. et al. Recommendations for neonatologist performed echocardiography in Europe: Consensus Statement endorsed by European Society for Paediatric Research (ESPR) and European Society for Neonatology (ESN). Pediatr. Res. 80, 465–471 (2016).
Mertens, L. et al. Targeted Neonatal Echocardiography in the Neonatal Intensive Care Unit: practice guidelines and recommendations for training. Writing Group of the American Society of Echocardiography (ASE) in collaboration with the European Association of Echocardiography (EAE) and the Association for European Pediatric Cardiologists (AEPC). J. Am. Soc. Echocardiogr. 24, 1057–1078 (2011).
El-Khuffash, A. et al. Deformation imaging and rotational mechanics in neonates: a guide to image acquisition, measurement, interpretation, and reference values. Pediatr. Res. 84, 30–45 (2018).
van Laere, D. et al. Application of NPE in the assessment of a patent ductus arteriosus. Pediatr. Res. 84, 46–56 (2018).
Groves, A. M. et al. Introduction to neonatologist-performed echocardiography. Pediatr. Res. 84, 1–12 (2018).
Patel, M. D. et al. Echocardiographic assessment of right ventricular afterload in preterm infants: maturational patterns of pulmonary artery acceleration time over the first year of age and implications for pulmonary hypertension. J. Am. Soc. Echocardiogr. 32, 884.e4–894.e4 (2019).
Levy, P. T. et al. Pulmonary artery acceleration time provides a reliable estimate of invasive pulmonary hemodynamics in children. J. Am. Soc. Echocardiogr. 29, 1056–1065 (2016).
de Boode, W. P. et al. Application of neonatologist performed echocardiography in the assessment and management of persistent pulmonary hypertension of the newborn. Pediatr. Res. 84, 68–77 (2018).
Wilson, J. R., Reichek, N. & Hirshfeld, J. Noninvasive assessment of load reduction in patients with asymptomatic aortic regurgitation. Am. J. Med. 68, 664–674 (1980).
Bruns, N. et al. How to administer near-infrared spectroscopy in critically ill neonates, infants, and children. J. Vis. Exp. https://doi.org/10.3791/61533 (2020).
Saugel, B. et al. Effects of red blood cell transfusion on hemodynamic parameters: a prospective study in intensive care unit patients. Scand. J. Trauma Resusc. Emerg. Med. 21, 21 (2013).
Baron-Stefaniak, J. et al. Transfusion of standard-issue packed red blood cells induces pulmonary vasoconstriction in critically ill patients after cardiac surgery-a randomized, double-blinded, clinical trial. PLoS ONE 14, e0213000 (2019).
Cambonie, G. et al. Myocardial adaptation to anemia and red blood cell transfusion in premature infants requiring ventilation support in the 1st postnatal week. Neonatology 92, 174–181 (2007).
Fredrickson, L. K. et al. Acute physiological effects of packed red blood cell transfusion in preterm infants with different degrees of anaemia. Arch. Dis. Child. Fetal Neonatal Ed. 96, F249–F253 (2011).
Yu, C. W., Sung, R. Y., Fok, T. F. & Wong, E. M. Effects of blood transfusion on left ventricular output in premature babies. J. Paediatr. Child Health 34, 444–446 (1998).
Radicioni, M., Troiani, S. & Mezzetti, D. Functional echocardiographic assessment of myocardial performance in anemic premature infants: a pilot study. Pediatr. Cardiol. 33, 554–561 (2012).
Saleemi, M. S. et al. Myocardial assessment using tissue doppler imaging in preterm very low-birth weight infants before and after red blood cell transfusion. J. Perinatol. 33, 681–686 (2013).
Alkalay, A. L. et al. Hemodynamic changes in anemic premature infants: are we allowing the hematocrits to fall too low? Pediatrics 112, 838–845 (2003).
Mintzer, J. P. et al. Monitoring regional tissue oxygen extraction in neonates <1250 g helps identify transfusion thresholds independent of hematocrit. J. Neonatal Perinat. Med. 7, 89–100 (2014).
Sandal, G. et al. Assessment of red blood cell transfusion and transfusion duration on cerebral and mesenteric oxygenation using near-infrared spectroscopy in preterm infants with symptomatic anemia. Transfusion 54, 1100–1105 (2014).
van Hoften, J. C. et al. Cerebral tissue oxygen saturation and extraction in preterm infants before and after blood transfusion. Arch. Dis. Child. Fetal Neonatal Ed. 95, F352–F358 (2010).
Dani, C. et al. Effect of blood transfusions on cerebral haemodynamics in preterm infants. Acta Paediatr. 91, 938–941 (2002).
Dani, C. et al. Blood transfusions increase cerebral, splanchnic, and renal oxygenation in anemic preterm infants. Transfusion 50, 1220–1226 (2010).
Poon, W. B. & Tagamolila, V. Cerebral perfusion and assessing hemodynamic significance for patent ductus arteriosus using near infrared red spectroscopy in very low birth weight infants. J. Matern. Fetal Neonatal Med. 34, 1645–1650 (2021).
Dix, L. et al. Cerebral oxygenation and echocardiographic parameters in preterm neonates with a patent ductus arteriosus: an observational study. Arch. Dis. Child. Fetal Neonatal Ed. 101, F520–F526 (2016).
Lemmers, P. M. et al. Patent ductus arteriosus and brain volume. Pediatrics 137, e20153090 (2016).
Cohen, E. et al. Reduction in cerebral oxygenation due to patent ductus arteriosus is pronounced in small-for-gestational-age neonates. Neonatology 111, 126–132 (2017).
Chock, V. Y., Rose, L. A., Mante, J. V. & Punn, R. Near-infrared spectroscopy for detection of a significant patent ductus arteriosus. Pediatr. Res. 80, 675–680 (2016).
Silverman, A. & Petersen, N. H. Physiology, Cerebral Autoregulation (StatPearls, 2021).
Ledo, A. et al. Abdominal near-infrared spectroscopy detects low mesenteric perfusion early in preterm infants with hemodynamic significant ductus arteriosus. Neonatology 112, 238–245 (2017).
Aktas, S. et al. Effects of blood transfusion on regional tissue oxygenation in preterm newborns are dependent on the degree of anaemia. J. Paediatr. Child Health 55, 1209–1213 (2019).
Banerjee, J., Leung, T. S. & Aladangady, N. Blood transfusion in preterm infants improves intestinal tissue oxygenation without alteration in blood flow. Vox Sang. 111, 399–408 (2016).
Bailey, S. M., Hendricks-Muñoz, K. D. & Mally, P. V. Variability in splanchnic tissue oxygenation during preterm red blood cell transfusion given for symptomatic anaemia may reveal a potential mechanism of transfusion-related acute gut injury. Blood Transfus. 13, 429–434 (2015).
Marin, T. et al. Red blood cell transfusion-related necrotizing enterocolitis in very-low-birthweight infants: a near-infrared spectroscopy investigation. Transfusion 53, 2650–2658 (2013).
MohanKumar, K. et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat. Commun. 10, 3494 (2019).
Rai, S. E., Sidhu, A. K. & Krishnan, R. J. Transfusion-associated necrotizing enterocolitis re-evaluated: a systematic review and meta-analysis. J. Perinat. Med. 46, 665–676 (2018).
Funding
The work received funding from the European Society for Paediatric Research (ESPR) Young Investigator START-UP Award 2019, Health Research Board Ireland [NCHF-2017-005] and The National Children’s Research Centre [D/17/7]. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Each author has met the Pediatric Research authorship requirements. A.S. obtained funding for the study, provided administrative, technical, and material support for the study, acquired, analysed and interpreted data and drafted the manuscript. S.A. provided administrative, technical, and material support for the study. E.D. provided critical revision of the manuscript for important intellectual content. A.E.-K. conceptualised and designed the study, obtained funding, provided administrative, technical, and material support for the study, acquired, analysed and interpreted data, performed the statistical analysis, drafted the manuscript and provided critical revision of the manuscript for important intellectual content and study supervision. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Informed written consent was obtained from all parents prior to enrolment.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Smith, A., Armstrong, S., Dempsey, E. et al. The impact of a PDA on tissue oxygenation and haemodynamics following a blood transfusion in preterm infants. Pediatr Res 93, 1314–1320 (2023). https://doi.org/10.1038/s41390-022-01967-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-01967-3