Abstract
Background
To examine whether oral administration of paracetamol as a first-line agent had a greater effect on the closure of a patent ductus arteriosus than the intravenous route.
Methods
We performed a retrospective study of preterm infants (<37 weeks of gestation) between 2012 and 2020 treated with oral or intravenous paracetamol as the first line for patent ductus arteriosus (PDA) constriction and compared rates of ductal closure, course duration, cumulative dose, PDA characteristics, and serum levels.
Results
Over the study period, 80 preterm infants received paracetamol, of which 50 received paracetamol as first-line treatment to augment constriction of the PDA. Closure rate was higher in the oral group (n = 15/19, 79%) compared to the intravenous group (n = 8/20, 40%, p < 0.01), and remained significant after adjusting for gestational age, length of treatment, and postnatal age (OR 0.14, 95% CI 0.03–0.67, p = 0.014, RR 0.51, 95% CI 0.28–0.91). Eleven preterm infants received a combination of both oral and intravenous paracetamol with a closure rate of 45% (n = 5).
Conclusions
Oral administration of paracetamol as a first-line agent is more efficacious to constrict the PDA than the intravenous route, irrespective of gestational age or course duration.
Impact
-
Our retrospective study comparing the use of oral versus intravenous paracetamol as the first line for patent ductus arteriosus (PDA) constriction in preterm infants demonstrates that oral administration of paracetamol is more efficacious to constrict the PDA than the intravenous route, irrespective of gestational age or course duration.
-
To our knowledge, this is the first published study (prospective or retrospective) to compare the efficacy of oral versus intravenous paracetamol as a first-line treatment for PDA closure in preterm infants.
-
Our finding may improve the rate of PDA closure when paracetamol is used as a first-line agent.
Similar content being viewed by others
Introduction
A patent ductus arteriosus (PDA) can have clinical consequences in extreme preterm infants based on the shunt burden, impact of cardiac performance, and associated perinatal characteristics that modulate the PDA.1,2,3 A hemodynamically significant PDA may exacerbate common morbidities of prematurity (e.g., bronchopulmonary dysplasia, necrotizing enterocolitis, retinopathy of prematurity, and intraventricular hemorrhage).2 Although there has been a recent trend for less aggressive treatment with the recognition that most PDAs may close spontaneously, a subgroup of preterm infants still likely benefit from treatment.2 In these situations, traditional first-line medical therapy consists of ibuprofen or indomethacin; two well-studied inhibitors of the enzyme cyclooxygenase (COX) that participate in prostaglandin H2 synthesis and augment closure of the PDA.
In 2011, Hammerman et al.4 first reported successful ductal closure with the administration of paracetamol to preterm infants who had failed or were contraindicated to receive indomethacin or ibuprofen. Since then, paracetamol has been extensively studied as a therapeutic alternative to COX-inhibitors.5,6,7 Paracetamol is thought to facilitate ductal closure via inhibition of the second active site on the prostaglandin H2 synthase, the peroxidase (POX) component.8 Paracetamol is a potentially appealing therapeutic choice with a less adverse side effect profile than indomethacin and ibuprofen. The success rate of ductal closure with paracetamol compared to indomethacin and ibuprofen is variably reported in the literature,6 in part because the prostaglandin inhibitory activity of paracetamol may be influenced by the route of administration (enteral vs. intravenous), dose, course length, and timing of administration.7,9,10
In 2012, our neonatal intensive care unit (NICU) began using paracetamol to augment closure of the PDA, and it is now considered a first-line treatment option. Although improved efficacy has been suggested for paracetamol administered orally compared with the intravenous route,7 variation in the route and duration of the courses still exists in our unit. Accordingly, the objective of this study was to investigate the impact of route of administration and cumulative dose on ductal closure in preterm infants receiving paracetamol as a first-line treatment for PDA. We hypothesized that oral administration would have a greater effect on ductal closure than the intravenous route.
Methods
Patient population and study design
This was a single-center retrospective study design of all preterm infants (born less than 32 weeks gestation) treated with oral or intravenous paracetamol as the first-line treatment to augment medical closure of the PDA from the neonatal intensive care unit (NICU) at Carmel Medical Center (Haifa, Israel) between January 2012 until June 2020. We excluded infants treated with paracetamol for other indications besides PDA closure (e.g., pain management), or infants who received paracetamol after having failed ibuprofen. The study was approved by the Carmel Medical Center Research Ethics Board.
PDA assessment
The standard of care in our unit proposes medical augmentation of PDA closure for only those infants with clinically and hemodynamically significant PDA.3 The need for PDA treatment is evaluated both clinically and by echocardiography. All echocardiography investigations were performed by a single pediatric cardiologist using ZONARE Z.One PRO:ZS3 ultrasound system, Mahwah, NJ. We acknowledge that it is challenging to characterize a hemodynamically significant PDA due to attributing clinical significance to varying degrees of respiratory and cardiovascular perturbations, but the “symptomatic treatment approach remains the most widely accepted clinical approach”.11 Hemodynamic and clinical assessment focused on (1) the PDA shunt volume appraisal and its impact on the systemic and pulmonary circulation (e.g., presence of a typical murmur, bounding femoral pulses, wide pulse pressure, worsening respiratory distress, evolving metabolic acidosis, and respiratory support); (2) myocardial function evaluation (e.g., hyperdynamic precordium, need for cardiotropic medications); and (3) perinatal shunt modifying characteristics (e.g., gestational age, growth restriction). We defined the PDA as small if the diameter was below 1.5 mm, moderate if between 1.5–2.0 mm, large >2 mm, and very large >3 mm.12 The PDA was considered hemodynamically significant in the presence of moderate to large PDA, coupled with evidence of shunt burden, myocardial compromise, and continued need for significant respiratory support.
Paracetamol course
All Infants received an oral or intravenous dose of 15 mg/kg paracetamol (Novimol Drops, C.T.S., 100 mg/ml, Israel or Paracetamol Taro I.V, 10 mg/ml, Neogen NV, Belgium, respectively). Oral doses were administered via the nasogastric tube with dilution (0.5 ml paracetamol in 2 ml water for injection) and the tube was flushed after the dose. Paracetamol was administered every 6 h, and the duration of treatment varied from a minimum of 3 days, and up to 7 days if ductal closure was not achieved after 3 days and the PDA was still considered hemodynamically significant. The attending neonatologists determined the initial route of administration. There was no uniform approach for the choice between the oral and intravenous route as the decision was at the discretion of the physician by personal preference. Infants who were not on enteral nutrition were always administered an intravenous dose. During a course, an infant could receive intravenous paracetamol, oral paracetamol, or a combination of both.
Outcome
The primary outcome was PDA closure, defined as a complete absence of a shunt on echocardiography at the end of the paracetamol course. Our unit practice was to perform echocardiograms at the end of each paracetamol course. Secondary outcomes included safety profile and common neonatal in-hospital mortality or morbidity (bronchopulmonary dysplasia, necrotizing enterocolitis, intraventricular hemorrhage, or retinopathy of prematurity, defined by commonly used published criteria).13,14,15
Other clinical and biochemical measures
We utilized a protocol to assess paracetamol levels and other clinical, biochemical, and imaging data. Serum paracetamol levels were tested in all cases after 24 h16 and determined using a colorimetric method (Cobas Integra®400 plus analyzer). We collected serum creatinine, hepatic transaminases, and bilirubin data prior to the initial use of paracetamol. Serum creatinine and hepatic transaminases were again taken on day 3 and at 5–7 days after the end of the course. Serum bilirubin level was obtained on day 3 of treatment. Total fluids administered per kg per day for the duration of the course were extracted from the charts.
Statistical analysis
Continuous variables are presented by the mean and standard deviation (STD) or median and interquartile range (IQR). The categorical variables are presented in percentages. The difference in demographical and clinical characteristics between the groups of administration route (oral, intravenous, or both) were analyzed using One way ANOVA, or Kruskal–Wallis, as appropriate for the continuous variables, and chi-square test for the categorical variables. Bonferroni correction was used for pairwise comparisons. Correlation between paracetamol route of administration and PDA closure was analyzed using logistic regression adjusted for all variables with p < 0.1 in the univariate model (gestational age, postmenstrual age, days of treatment, and total fluids per day). Odds ratios (OR) and relative risks (RR) with 95% CI are presented. The generalized linear model with binomial distribution and log link function was used to compute the relative risk using the PROC GENMOD procedure in SAS version 9.4. All other analyses were performed using IBM statistics software (SPSS 24). p < 0.05 was considered statistically significant.
Results
Patient characteristics
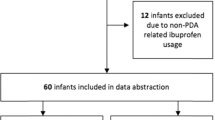
Eighty preterm newborns received paracetamol from January 2012 to June 2020. Twenty-nine patients received paracetamol for pain management and one patient received paracetamol after administration of ibuprofen as first-line therapy to augment closure of the PDA and were excluded from the patient cohort. Fifty preterm infants received a course of paracetamol as the first-line treatment for PDA closure without any previous exposure to non-steroidal anti-inflammatory drugs (Fig. 1). Median (interquartile range) gestational age and birth weight for the cohort was 27 weeks (25, 28) and 873 g (654, 1081), respectively. Eighty-four percent (n = 42) were treated within the first week of age and 94% (n = 47) were treated within the first 10 days of age. The duration of the course varied between 3 and 7 days (median, IQR [4, 3–5]). More than half (n = 36, 72%) of the patients received >3 days of treatment. Daily total fluids given were significantly higher in the non-closure group. This difference remained significant after adjustment for potential confounders (odds ratio, OR 0.96, 95% confidence intervals, CI 0.91–0.97, p = 0.033, RR 0.98 (0.97–0.99)). The overall survival rate to the discharge of the entire cohort was 84% (n = 42) with one death during the initial course of paracetamol treatment and the other deaths unrelated to the PDA (Table 1). None of the cases had liver dysfunction or significant hyperbilirubinemia during or after the course.
PDA characteristics
In 28 (56%) patients, PDA closure was achieved after the paracetamol course. Of these 28, 14% (n = 4) reopened their PDA and were treated with further medical and/or surgical interventions (Fig. 1). In 22 (44%) patients, ductal closure was not achieved after the initial course of paracetamol. In 7 of these patients (36%), the duct was small, and no other measures were initially taken to augment closure. One patient with a small duct after initial treatment became clinically symptomatic and a repeat echocardiogram revealed a large PDA; a second paracetamol course was administered with ductal closure confirmed. Fourteen patients (64%) who received an initial course without closure had a moderate to the large duct (Table 2). Two infants died early, one during the course with pulmonary hemorrhage, severe intraventricular hemorrhage, and intestinal perforation and another shortly after completion of a 5-day course of paracetamol from tracheal perforation while awaiting ligation. Further interventions for infants who failed closure following the first course included an additional paracetamol course, an additional ibuprofen course, or PDA ligation.
Paracetamol administration route
Nineteen infants (38%) received only oral paracetamol, 20 (40%) received only intravenous paracetamol and 11 infants (22%) received both intravenous and oral paracetamol doses within the same course. Closure and clinical characteristics by administration route are presented in Table 3. The closure rate after the first course for the oral group was 79% (15/19) compared to 40% (8/20) for the intravenous group, (p < 0.01). The difference remained significant after adjusting for potential confounders (OR 0.14 95% CI 0.03–0.67, p = 0.014, RR 0.51 95% CI (0.28–0.91)), which included all variables with p < 0.1 in the univariate model: gestational age, total days of treatment, daily total fluids, and postmenstrual age. There was no significant difference between the oral and intravenous groups in gestational age, birth weight, size of PDA prior to treatment, postnatal age at the beginning of the treatment, nutritional support status, or presence of any IVH. The intravenous-only group received significantly longer treatments and cumulative dose. There was no significant difference between the oral and intravenous groups in rates of bronchopulmonary dysplasia, necrotizing enterocolitis, and retinopathy of prematurity.
Discussion
The use of paracetamol to facilitate ductal closure via an alternate pathway of prostaglandin inhibition has rapidly grown over the last decade.5,6 We performed a descriptive retrospective cohort study of our single-center experience with paracetamol administration as a first-line agent in the first week of age and found a significantly higher rate of PDA closure with oral administration of paracetamol (78.9%) compared to the intravenous route (40%). This difference was not affected by gestational age, duration of the course, cumulative dose, size of the PDA prior to treatment, or by postnatal age at the beginning of the course. The decision to augment closure of the PDA with medical therapy remains an individualized targeted approach based on the clinical and hemodynamic presentation, but this study highlights the importance of considering the route of administration for paracetamol use in preterm born infants.
To our knowledge, this is the first published study (prospective or retrospective) to compare the efficacy of oral versus intravenous paracetamol as a first-line treatment for PDA closure in preterm infants. The current literature on paracetamol efficacy is inconsistent and based on a combination of small cases series, retrospective investigations, uncontrolled studies, or retrospective studies.5 While improved efficacy was suggested for paracetamol administered orally compared with the intravenous route from data derived from uncontrolled studies,7 the majority of data comes from infants who also received a prior course of ibuprofen or indomethacin. For example, Sancak et al.17 found a higher rate of overall PDA closure in a group of preterm infants receiving oral paracetamol group (n = 10, 88%) versus intravenous paracetamol group (n = 8, 70%), but the differences were not significant, and several patients received paracetamol as second-line therapy in this study. In a small case series of 21 patients, El-Khuffash et al.10 retrospectively evaluated 21 infants who received one of three paracetamol regimens: a 2 day (n = 5) or 7 day (n = 7) course of oral paracetamol or an intravenous paracetamol course for 2–6 days (n = 9). The majority (76%) failed an initial course on indomethacin. Infants who received intravenous paracetamol had the highest ductal closure rate, whereas none of the infants who received the short oral course showed any response to treatment. All infants receiving the long oral paracetamol course, except one, constricted their ductus and required no further intervention. In contrast, Vaidya et al. observed a higher rate of PDA closure following intravenous administration (46% vs. 31%), but close to 60% of this population initially received indomethacin for IVH prophylaxis or the first line PDA treatment.18
Enhanced efficacy of oral administration of other medications to constrict the PDA compared to intravenous administration have been previously demonstrated.18,19 In a recent Cochrane review18 of five studies with 406 infants, intravenous ibuprofen was significantly less efficacious than oral ibuprofen (RR 0.38, 95% CI 0.26–0.56). Similar findings were observed in a network meta-analysis19 where multiple treatment choices and regimens were compared; oral ibuprofen was superior to two intravenous therapies (ibuprofen and indomethacin). Oral paracetamol also consistently ranked high across all effectiveness outcomes.19 The difference between oral and intravenous administration may depend on the steadier plasma levels of the drug administered orally, similarly to what was observed for the oral use of ibuprofen.6,19,20 Barzilai et al.20 demonstrated that the maximal concentration reached after an oral dose of Ibuprofen was lower than in the intravenous route, however, the area under the curve (AUC0→24) was greater compared to the intravenous route, perhaps contributing to the better closure rate. Pacifici21 confirmed the higher effectiveness of oral over intravenous ibuprofen and hypothesized that a slower absorption rate and a longer half-life prolong the time of contact with the PDA, leading to a better response rate. Anderson et al.22 performed a pooled analysis on the pharmacokinetic properties of paracetamol and found the absorption half-life for oral paracetamol was prolonged in newborns compared to older infants, and absorption was further delayed in premature neonates during the first days of life. Slower gastric emptying in the neonate as well as a nearly neutral gastric pH, which contributes to higher availability of the unionized form may increase absorption.22
The rate of PDA closure following administration of paracetamol is difficult to discern from the current literature for several reasons. First, the definition of closure and PDA assessment varies between studies with some defining closure as complete closure, others <0.5 mm, and some using a more subjective definition of lack of hemodynamic significance.5,6,23 Second, many studies group the intravenous and oral routes together. Third, while the rates of closure for the intravenous route ranges between 57 and 100%10,23,24,25 and the rates of closure for the oral route ranges between 65 and 100%,4,26,27,28 there is considerable variation in sample sizes, postnatal age at the beginning of the treatment, and duration of courses in each of these studies. A reduced efficacy for paracetamol compared to ibuprofen and indomethacin has been demonstrated in uncontrolled studies for preterm infants <26–28 weeks gestation.16,29 On the other hand, all three medications used to constrict the PDA seem to be equally efficacious (60–70%) in infants >28 weeks gestation. In our study, the observed differences in closure rates between the oral and intravenous routes were not affected by gestational age. We suspect that our observations may be explained by our study design focused on the use of paracetamol as a first-line agent and often treatment initiated early (during the week of age), at a time with higher circulating levels of prostaglandins that eventually decrease with increasing postnatal age.30
There is a large body of evidence regarding the use of paracetamol with regards to its efficacy and safety profile comparisons to ibuprofen and indomethacin.6 A recent meta-analysis of 8 studies6 including 916 infants comparing paracetamol to ibuprofen and indomethacin showed similar closure rates between the drugs. There was no difference in mortality, reopening of the ductus, need for surgical closure, rate of intraventricular hemorrhage, periventricular leukomalacia, necrotizing enterocolitis, or bronchopulmonary dysplasia between the groups. However, there was a significant difference between the paracetamol and the ibuprofen groups in gastrointestinal bleeding favoring paracetamol over ibuprofen, and a significant difference in creatinine levels, urine output, and platelet count favoring paracetamol over ibuprofen and indomethacin. Coupled with our observations and others,19 oral paracetamol may be as effective as ibuprofen or indomethacin in closing the PDA with less adverse effects.
In our study, closure rate was not associated with trough paracetamol levels or with cumulative dose given. McPherson et al. found no association between paracetamol steady-state serum concentrations and ductal closure during an intravenous course.31 In contrary, Bin-Nun et al.9 found a significant positive correlation between serum levels during oral treatment and ductal response in the small series, and El-Khuffash et al.10 found a significant difference in closure rate between a short and a long course. Future large prospective pharmacokinetic studies carefully designed to evaluate the AUC0→24 and dose-response relationship of oral versus intravenous paracetamol are needed to clarify these questions.
The significance of these findings should be interpreted within the framework of this retrospective study’s limitations. Causality cannot be proven from the retrospective, nonrandomized design, and the association between the oral route and PDA closure may be related to chance, random error, bias, or other confounding factors. Although we were able to control for several potential confounders, such as paracetamol serum level, cumulative dose, and fluid restriction during the course, a prospective controlled clinical and pharmacokinetic study is needed to confirm our results before recommendations for paracetamol treatment can be made. In our cohort, total daily-administered fluid volume per kilograms was lower in the infants who achieved ductal closure. Although fluid restriction has been shown to impact PDA hemodynamics,31,32 recent data suggests an adverse impact on cardiac output and nutrition with little impact on respiratory mechanics following fluid restriction.33,34 We could not account for all the biases of the attending physicians. Interestingly, the duration and cumulative dose were longer and higher, respectively, in the intravenous group, but the closure rate was higher in the oral group. The typical decision to prolong the course was made when ductal closure was not achieved after the completion of 3 days. Although there was no standardized approach for which patients would be treated with oral or intravenous paracetamol, the typical practice was to start oral administration in patients tolerating enteral nutrition. Finally, the relatively small sample and the lack of validated echocardiographic criteria for defining the hemodynamics significance may limit the generalizability of the data, but provides proof of concept for future prospective analysis.
Conclusion
Paracetamol is a recognized alternative for the closure of a PDA in extreme preterm infants. For the first time, we demonstrate that oral administration of paracetamol as a first-line agent is more efficacious to constrict the PDA than the intravenous administration of paracetamol, irrespective of gestational age or course duration. Future work is needed to validate these findings and explore the pharmacokinetics to decipher the mechanistic underpinnings.
References
Noori, S. et al. Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics 123, e138–e144 (2009).
Hamrick, S. E. et al. Patent ductus arteriosus of the preterm infant. Pediatrics 146, e20201209 (2020).
El-Khuffash, A., Levy, P. T., Gorenflo, M. & Frantz, I. D. 3rd The definition of a hemodynamically significant ductus arteriosus. Pediatr. Res. 85, 740–741 (2019).
Hammerman, C. et al. Ductal closure with paracetamol: a surprising new approach to patent ductus arteriosus treatment. Pediatrics 128, e1618–e1621 (2011).
Jasani, B., Weisz, D. E. & McNamara, P. J. Evidence-based use of acetaminophen for hemodynamically significant ductus arteriosus in preterm infants. Semin. Perinatol. 42, 243–252 (2018).
Ohlsson, A. & Shah, P. S. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. Cochrane Database Syst Rev. 27, CD010061 (2020).
Terrin, G. et al. Paracetamol for the treatment of patent ductus arteriosus in preterm neonates: a systematic review and meta-analysis. Arch. Dis. Child Fetal Neonatal Ed. 101, F127–36 (2016).
Ferguson, J. M. Pharmacotherapy for patent ductus arteriosus closure. Congenit. Heart Dis. 14, 52–56 (2019).
Bin-Nun, A., Fink, D., Mimouni, F. B., Algur, N. & Hammerman, C. Paracetamol serum concentrations in neonates treated enterally for ductal closure: a pilot study. J. Pediatr. 198, 304–307 (2018).
El-Khuffash, A. et al. Efficacy of paracetamol on patent ductus arteriosus closure may be dose dependent: evidence from human and murine studies. Pediatr. Res. 76, 238–44 (2014).
Vaidya, R., Knee, A., Paris, Y. & Singh, R. Predictors of successful patent ductus arteriosus closure with acetaminophen in preterm infants. J. Perinatol. 41, 998–1006 (2021).
Ramos, F. G., Rosenfeld, C. R., Roy, L., Koch, J. & Ramaciotti, C. Echocardiographic predictors of symptomatic patent ductus arteriosus in extremely-low- birth-weight preterm neonates. J. Perinatol. 30, 535–9 (2010).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatr. 92, 529–34 (1978).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med 163, 1723–9 (2001).
Bell, M. J. et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 187, 1–7 (1978).
Allegaert, K., Palmer, G. M. & Anderson, B. J. The pharmacokinetics of intravenous paracetamol in neonates: size matters most. Arch. Dis. Child. 96, 575–80 (2011).
Sancak, S. et al. Oral versus intravenous paracetamol: which is better in closure of patent ductus arteriosus in very low birth weight infants? J. Matern. Fetal Neonatal Med. 29, 135–139 (2016).
Ohlsson, A., Walia, R. & Shah, S. S. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst. Rev. 11, CD003481 (2020).
Mitra, S. et al. Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. JAMA 319, 1221–1238 (2018).
Barzilay, B. et al. Pharmacokinetics of oral ibuprofen for patent ductus arteriosus closure in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 97, F116–9 (2012).
Pacifici, G. M. Clinical pharmacology of ibuprofen in preterm infants: a meta-analysis of published data. http://www.gnresearch.org/doi/10.5935/MedicalExpress.2014.02.02. Accessed 10 August 2020 (2014).
Anderson, B. J., Woollard, G. A. & Holford, N. H. A model for size and age changes in the pharmacokinetics of paracetamol in neonates, infants and children. Br. J. Clin. Pharmacol. 50, 125–134 (2000).
Valerio, E. et al. Intravenous paracetamol for PDA closure in the preterm: a single-center experience. Eur. J. Pediatr. 175, 953–966 (2016).
Terrin, G. et al. Efficacy of paracetamol for the treatment of patent ductus arteriosus in preterm neonates. Ital. J. Pediatr. 20, 21 (2014).
Oncel, M. Y. et al. Intravenous paracetamol treatment in the management of patent ductus arteriosus in extremely low birth weight infants. Neonatology 103, 166–9 (2013).
Ghaderian, M., Barekatain, B. & Dardashty, A. B. Comparison of oral acetaminophen with oral ibuprofen on closure of symptomatic patent ductus arteriosus in preterm neonates. J. Res. Med. Sci. 24, 96 (2019).
Balachander, B. et al. Comparison of efficacy of oral paracetamol versus ibuprofen for PDA closure in preterms—a prospective randomized clinical trial. J. Matern. Fetal Neonatal Med. 33, 1587–1592 (2020).
Yurttutan, S. et al. A different first-choice drug in the medical management of patent ductus arteriosus: oral paracetamol. J. Matern. Fetal Neonatal Med. 26, 825–7 (2013).
Liebowitz, M. et al. Comparative effectiveness of drugs used to constrict the patent ductus arteriosus: a secondary analysis of the PDA-TOLERATE trial. J. Perinatol. 39, 599–607 (2019).
Clyman, R. I. Recommendations for the postnatal use of indomethacin: an analysis of four separate treatment strategies. J. Pediatr. 128, 601–7 (1996).
McPherson, C., Luecke, C. M., Liviskie, C. J., Zeller, B. N. & Vesoulis, Z. A. Acetaminophen serum concentrations in infants treated intravenously for patent ductus arteriosus. J. Pediatr. Pharm. Ther. 24, 134–137 (2019).
Bell, E. F. & Acarregui, M. J. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 12, CD000503 (2014).
Hansson, L., Lind, T., Wiklund, U., Öhlund, I. & Rydberg, A. Fluid restriction negatively affects energy intake and growth in very low birthweight infants with haemodynamically significant patent ductus arteriosus. Acta Paediatr. 108, 1985–1992 (2019).
De Buyst, J., Rakza, T., Pennaforte, T., Johansson, A. B. & Storme, L. Hemodynamic effects of fluid restriction in preterm infants with significant patent ductus arteriosus. J. Pediatr. 161, 404–8 (2012).
Author information
Authors and Affiliations
Contributions
G.A.: study conception and design, acquisition of data, analysis and interpretation of data, and the first draft of the manuscript. P.T.L./R.A./G.M./M.M./L.-N.K.: substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, revising the article critically for important intellectual content, and final approval of the version to be published. K.I.: study design, acquisition of data, revising the article critically for important intellectual content, and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Patient consent was not required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gover, A., Levy, P.T., Rotschild, A. et al. Oral versus intravenous paracetamol for patent ductus arteriosus closure in preterm infants. Pediatr Res 92, 1146–1152 (2022). https://doi.org/10.1038/s41390-022-01944-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-01944-w
This article is cited by
-
Acetaminophen for the patent ductus arteriosus: has safety been adequately demonstrated?
Journal of Perinatology (2023)
-
A perspective on the potential side effects of paracetamol use in the treatment of PDA
Pediatric Research (2022)