Abstract
Background
To determine the association of gestational age (GA) and day of life (DOL) with the circulating serum concentration of six brain injury-associated biomarkers in non-brain injured neonates born between 23 and 41 weeks’ GA.
Methods
In a multicenter prospective observational cohort study, serum CNS-insult, inflammatory and trophic proteins concentrations were measured daily in the first 7 DOL.
Results
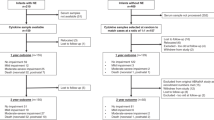
Overall, 3232 serum samples were analyzed from 745 enrollees, median GA 32.3 weeks. BDNF increased 3.7% and IL-8 increased 8.9% each week of gestation. VEGF, IL-6, and IL-10 showed no relationship with GA. VEGF increased 10.8% and IL-8 18.9%, each DOL. IL-6 decreased by 15.8% each DOL. IL-10 decreased by 81.4% each DOL for DOL 0-3. BDNF did not change with DOL. Only 49.67% of samples had detectable GFAP and 33.15% had detectable NRGN. The odds of having detectable GFAP and NRGN increased by 53% and 11%, respectively, each week after 36 weeks’ GA. The odds of having detectable GFAP and NRGN decreased by 15% and 8%, respectively, each DOL.
Conclusions
BDNF and IL-8 serum concentrations vary with GA. VEGF and interleukin concentrations are dynamic in the first week of life, suggesting circulating levels should be adjusted for GA and DOL for clinically relevant assessment of brain injury.
Impact
-
Normative data of six brain injury-related biomarkers is being proposed.
-
When interpreting serum concentrations of brain injury biomarkers, it is key to adjust for gestational age at birth and day of life during the first week to correctly assess for clinical brain injury in neonates.
-
Variation in levels of some biomarkers may be related to gestational and postnatal age and not necessarily pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Fenichel, G. M. Hypoxic-ischemic encephalopathy in the newborn. Arch. Neurol. 40, 261–266 (1983).
Chin, E. M. et al. Preschool language outcomes following perinatal hypoxic-ischemic encephalopathy in the age of therapeutic hypothermia. Dev. Neurosci. 40, 627–637 (2019).
Kurinczuk, J. J., White-Koning, M. & Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic – ischaemic encephalopathy. Early Hum. Dev. 86, 329–338 (2010).
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 353, 1574–1584 (2005).
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8, 110–124 (2009).
Bolisetty, S. et al. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics 133, 55–62 (2014).
Chevallier, M. et al. Leading causes of preterm delivery as risk factors for intraventricular hemorrhage in very preterm infants: results of the EPIPAGE 2 cohort study. Am. J. Obstet. Gynecol. 216, 518.e1 (2017).
Leviton, A. & Paneth, N. White matter damage in preterm newborns—an epidemiologic perspective. Early Hum. Dev. 24, 1–22 (1990).
Sarnat, H. B. & Sarnat, M. S. Encephalopathy fetal distress a clinical and electroencephalographic study. Arch. Neurol. 33, 696–705 (1976).
Edwards, A. D. et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 340, 409 (2010).
Shankaran, S. et al. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy a randomized clinical trial. JAMA - J. Am. Med Assoc. 318, 57–67 (2017).
American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Neonatal encephalopathy and neurologic outcome, second edition. Obstet. Gynecol. 123, 896–901 (2014).
Gerner, G. J. et al. Correlation between white matter injury identified by neonatal diffusion tensor imaging and neurodevelopmental outcomes following term neonatal asphyxia and therapeutic hypothermia: an exploratory pilot study. J. Child Neurol. 34, 556–566 (2019).
Salas, J., Reddy, N., Carson, K. A., Northington, F. J. & Huisman, T. A. G. M. Ultrasound predicts white matter integrity after hypothermia therapy in neonatal hypoxic-ischemic injury. J. Neuroimaging 29, 743–749 (2019).
Blennow, K. A review of fluid biomarkers for Alzheimer’s disease: moving from CSF to blood. Neurol. Ther. 6, 15–24 (2017).
Pelinka, L. E. et al. GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J. Neurotrauma 21, 1553–1561 (2004).
Missler, U., Wiesmann, M., Wittmann, G., Magerkurth, O. & Hagenström, H. Measurement of glial fibrillary acidic protein in human blood: analytical method and preliminary clinical results. Clin. Chem. 44, 138–141 (1999).
Ennen, C. S. et al. Glial fibrillary acidic protein as a biomarker for neonatal hypoxic-ischemic encephalopathy treated with whole-body cooling. Am. J. Obstet. Gynecol. 205, 251.e1–251.e7 (2011).
Blennow, M., Hagberg, H. & Rosengren, L. Glial fibrillary acidic protein in the cerebrospinal fluid: a possible indicator of prognosis in full-term asphyxiated newborn infants? Pediatr. Res 37, 260–264 (1995).
Stewart, A. et al. Glial fibrillary acidic protein as a biomarker for periventricular white matter injury. Am. J. Obstet. Gynecol. 209, 27.e1–27.e7 (2013).
Chalak, L. F. et al. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J. Pediatr. 164, 468–74.e1 (2014).
Massaro, A. N. et al. Serum biomarkers of MRI brain injury in neonatal hypoxic ischemic encephalopathy treated with whole-body hypothermia: a pilot study. Pediatr. Crit. Care Med. 14, 310–317 (2013).
Blennow, M., Savman, K., Ilves, P., Thoresen, M. & Rosengren, L. Brain-specific proteins in the cerebrospinal fluid of severely asphyxiated newborn infants. Acta Paediatr. 90, 1171–1175 (2001).
Yang, J., Korley, F. K., Dai, M. & Everett, A. D. Serum neurogranin measurement as a biomarker of acute traumatic brain injury. Clin. Biochem 48, 843–848 (2015).
Çevik, S. et al. NRGN, S100B and GFAP levels are significantly increased in patients with structural lesions resulting from mild traumatic brain injuries. Clin. Neurol. Neurosurg. 183, 105380 (2019).
Represa, A. et al. Neurogranin: immunocytochemical localization of a brain-specific protein kinase C substrate. J. Neurosci. 10, 3782–3792 (1990).
Guadaño-Ferraz, A., Viñuela, A., Oeding, G., Bernal, J. & Rausell, E. RC3/neurogranin is expressed in pyramidal neurons of motor and somatosensory cortex in normal and denervated monkeys. J. Comp. Neurol. 493, 554–570 (2005).
Dietrick, B. et al. Plasma and cerebrospinal fluid candidate biomarkers of neonatal encephalopathy severity and neurodevelopmental outcomes. J. Pediatr. 226, 71–79.e5 (2020).
Massaro, A. N. et al. Plasma biomarkers of brain injury in neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 194, 67–75 (2018).
Çelik, Y. et al. The effects of selective head cooling versus whole-body cooling on some neural and inflammatory biomarkers: a randomized controlled pilot study. Ital. J. Pediatr. 41, 1–8 (2015).
Chaparro-Huerta, V. et al. Proinflammatory cytokines, enolase and S-100 as early biochemical indicators of hypoxic-ischemic encephalopathy following perinatal asphyxia in newborns. Pediatr. Neonatol. 58, 70–76 (2017).
Barde, Y. A., Edgar, D. & Thoenen, H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1, 549–553 (1982).
Korte, M. et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl Acad. Sci. USA 92, 8856–8860 (1995).
Leal, G., Comprido, D. & Duarte, C. B. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 76, 639–656 (2014).
Park, H. & Poo, M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 14, 7–23 (2012).
Grad, S. et al. Strongly enhanced serum levels of vascular endothelial growth factor (VEGF) after polytrauma and burn. Clin. Chem. Lab Med 36, 379–383 (1998).
Lv, H. et al. Neonatal hypoxic ischemic encephalopathy-related biomarkers in serum and cerebrospinal fluid. Clin. Chim. Acta 450, 282–297 (2015).
Sweetman, D. U., Onwuneme, C., Watson, W. R., Murphy, J. F. A. & Molloy, E. J. Perinatal asphyxia and erythropoietin and VEGF: serial serum and cerebrospinal fluid responses. Neonatology 111, 253–259 (2017).
Graham, E. M., Everett, A. D., Delpech, J. C. & Northington, F. J. Blood biomarkers for evaluation of perinatal encephalopathy: state of the art. Curr. Opin. Pediatr. 30, 199–203 (2018).
Ramaswamy, V. et al. Systematic review of biomarkers of brain injury in term neonatal encephalopathy. Pediatr. Neurol. 40, 215–226 (2009).
Broni, E. K. et al. Blood biomarkers for neonatal hypoxic – ischemic encephalopathy in the presence and absence of sentinel events. J. Perinatol. https://doi.org/10.1038/s41372-020-00850-5 (2020).
Novak, C. M., Eke, A. C., Ozen, M., Burd, I. & Graham, E. M. Risk factors for neonatal hypoxic-ischemic encephalopathy in the absence of sentinel events. Am. J. Perinatol. 36, 27–33 (2019).
Schleif, W. et al. Tiny bodies, big needs: prospective biobanking of neonatal clinical remnant samples. Biopreserv. Biobank. 19, 106–110 (2021).
Shimada, H. et al. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Front. Aging Sci. 6, 1–9 (2014).
Martínez-Moreno, A., Rivera-Olvera, A. & Escobar, M. L. BDNF induces in vivo long-lasting enhancement of synaptic transmission and structural reorganization at the hippocampal mossy fibers in a transcription and translation-independent manner. Neurobiol. Learn. Mem. 167, 107125 (2020).
Edelmann, E., Leßmann, V. & Brigadski, T. Pre- and postsynaptic twists in BDNF secretion and action in synaptic plasticity. Neuropharmacology 76, 610–627 (2014).
Panja, D. & Bramham, C. R. BDNF mechanisms in late LTP formation: a synthesis and breakdown. Neuropharmacology 76, 664–676 (2014).
Tolwani, R. J. et al. BDNF overexpression produces a long-term increase in myelin formation in the peripheral nervous system. J. Neurosci. Res. 77, 662–669 (2004).
Karege, F., Schwald, M. & Cisse, M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci. Lett. 328, 261–264 (2002).
Sartorius, A. et al. Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry 42, 270–276 (2009).
Klein, A. B. et al. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J. Neuropsychopharmacol. 14, 347–353 (2011).
Graham, E. M. et al. Association of intraoperative circulating-brain injury biomarker and neurodevelopmental outcomes at 1 year among neonates who have undergone cardiac surgery. J. Thorac. Cardiovasc. Surg. 157, 1996–2002 (2019).
Easley, R. B. et al. Impaired cerebral autoregulation and elevation in plasma glial fibrillary acidic protein level during cardiopulmonary bypass surgery for CHD. Cardiol. Young. 28, 55–65 (2018).
Guo, J. H. et al. Expression pattern of NeuN and GFAP during human fetal spinal cord development. Child’s Nerv. Syst. 31, 863–872 (2015).
Nazir, F. H. et al. Expression and secretion of synaptic proteins during stem cell differentiation to cortical neurons. Neurochem. Int. 121, 38–49 (2018).
Romero, R. et al. Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. J. Perinat. Med. 44, 53–76 (2016).
Goepfert, A. R. et al. Umbilical cord plasma interleukin-6 concentrations in preterm infants and risk of neonatal morbidity. Am. J. Obstet. Gynecol. 191, 1375–1381 (2004).
Sood, B. G., Madan, A., Saha, S., Schendel, D. & Thorsen, P. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr. Res. 67, 394–400 (2010).
Acknowledgements
We thank the patients and their families for their participation and contributions to this study and the Everett research group at Johns Hopkins University School of Medicine for their support and contributions and the Johns Hopkins All Children’s Pediatric Biorepository for its support. We also thank the Johns Hopkins University School of Medicine Scholarly Concentration mentor Dr. Meredith Atkinson and the Johns Hopkins University School of Medicine Dean’s Funding for their support and contributions.
Funding
This study was supported by NIH NICHD R01HD086058 [A.E., F.N., J.Y., D.V., S.B., and E.G.], NINDS KO8NS096115 [R.C.-V.], Thomas-Wilson Foundation [R.C.-V.], and JHACH Foundation Institutional Research Grant Program [S.B.].
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: S.B., B.D.F., F.N., E.G., A.T., J.Z.B.S., D.V., and A.D.E. Drafting the article or revising it critically for important intellectual content: S.B., B.D.F., F.N., E.G., A.T., V.J.B., G.G., R.C.-V., D.V., and A.D.E. Final approval of the version to be published: S.B., B.D.F., F.N., E.G., R.C.-V., D.V., and A.D.E.
Corresponding author
Ethics declarations
Competing interests
Under a license agreement between ImmunArray Ltd. and Johns Hopkins University, the University and Dr. Everett are entitled to royalties on an invention described in this study and discussed in this publication. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies. Sandra Brooks and Barbara Dietrick wrote the first draft of the manuscript, and no honorarium, grant, or other forms of payment was given to anyone to produce the manuscript. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Brooks, S., Friedes, B.D., Northington, F. et al. Serum brain injury biomarkers are gestationally and post-natally regulated in non-brain injured neonates. Pediatr Res 93, 1943–1954 (2023). https://doi.org/10.1038/s41390-021-01906-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01906-8