Abstract
Introduction
Variability of arterial blood pressure (ABP) has been associated with intraventricular hemorrhage in very preterm neonates (VPT) and may predict other brain lesions assessed at term-equivalent of age (TEA).
Methods
This was a prospective single-center study including VPT with early invasive continuous ABP monitoring and assessed at TEA using brain magnetic resonance imaging (TEA-MRI). The association between early mean ABP (MABP) and TEA-MRI findings was modeled by multivariate logistic regression analysis using covariates selected by the LASSO method.
Results
Among 99 VPT, the LASSO procedure selected consecutive periods of lowest MABP of 30 min on day 1 (d1) and 10 min on day 2 (d2) as the most relevant durations to predict TEA-MRI findings (OR [95% CI], 1.11 [1.02–1.23], p = 0.03 and 1.13 [1.01–1.27], p = 0.03, respectively). ROC curve analysis showed optimal thresholds at 30.25 mmHg on d1 and 33.25 mmHg on d2. This significant association persisted after adjustment with covariates including birthweight, gestational age, sex, and inotrope exposure. Final models selected by LASSO included the decile of the birthweight and lowest MABP for 30 min on d1 and 10 min on d2, for which the areas under the ROC curve were 74% and 75%, respectively.
Conclusion
Early continuous ABP monitoring may predict brain TEA-MRI findings in VPT.
Impact
-
Early arterial blood pressure monitoring may contribute to predicting brain damage upon MRI at term-equivalent of age for infants born very preterm.
-
Careful blood pressure continuous monitoring in very preterm infants may identify infants at risk of long-term brain damage.
-
Umbilical artery catheterization provides the best option for continuously monitoring arterial blood pressure in very preterm infants.
Similar content being viewed by others
Introduction
Hemodynamic adaptation early after birth is considered to be crucial for the regulation of cerebral perfusion and the prevention of brain injury in preterm infants.1 The causal relationship between arterial blood pressure (ABP) and brain injury is yet to be fully demonstrated, but systemic low or unstable ABP has already been shown to be associated with the occurrence of intraventricular hemorrhage (IVH) in premature infants.2,3 More recently, variability in early continuous ABP recordings was also shown to correlate with the development of IVH within the first postnatal week, with a key role of a wide range in extreme values.4,5,6 In addition, early changes in ABP and antihypotensive therapy were found to increase the risk of IVH, death, or neurodevelopmental impairment in extremely preterm infants.7,8 It has been long accepted that hemodynamically mediated brain hemorrhage in the premature infant is related to an immature cardiovascular system acting on a fragile cerebral vasculature with immature intrinsic cerebral autoregulation.9 Aside from IVH, the effect of fluctuations in ABP on the early vulnerability of the developing brain is yet to be studied. In particular, these effects might play a role in white matter (WM) injury, a key component of the encephalopathy of prematurity.10 As neurodevelopmental handicaps observed in infants born preterm are a major concern, early predictors may help physicians in providing personalized care and parental counseling. Here, we hypothesized that a low or unstable mean ABP (MABP) soon after birth may be associated with brain lesions, as assessed at term-equivalent of age (TEA) by magnetic brain imaging (MRI).
In this pilot study, we investigated whether invasive ABP continuous monitoring during the first postnatal days can predict TEA-MRI findings in infants born very preterm and the lowest MABP associated with the subsequent development of brain lesions.
Patients and methods
Patients
This study is a retrospective analysis of data prospectively collected from surviving infants born before 286/7 weeks of gestation at the Geneva University Hospital by invasive ABP continuous monitoring during the first three postnatal days and assessed at TEA by brain MRI. The study was approved by the Swiss National Ethics Committee (BASEC N°2018-00738). A form providing written consent was collected from the parents of all patients at admission.
Blood pressure data collection
All ABP measurements were invasively collected by umbilical artery catheterization (2.5 Fr/30 cm umbilical catheter, Vygon, Ecouen, France), with the catheter generally placed high in the aorta (T6 to T10), used in our unit as a standard of care for very preterm infants. In our local guidelines, all infants below 28 completed weeks or weighing less than 1000 g should undergo umbilical artery catheterization for blood sampling and ABP monitoring. This arterial line provides continuous measurement of systolic and diastolic blood pressure and mean arterial blood pressure (MABP). The cannula is connected to an infusion set fitted with a transducer. The device is zeroed when the air–fluid interface is opened to atmospheric pressure at the level of the infant’s heart. Interventions to treat low ABP were generally initiated upon observation of a MABP lower than that appropriate for the gestational age of the infant associated with other evidence of reduced organ perfusion (reduced urine output, lethargy, abnormal skin color, etc.). Either volume expanders or inotropes (dopamine) were used as first-line treatments.
ABP values were prospectively collected within the first 72 postnatal hours from the patient monitor (Philips MP70) and stored in a local database using Centricity Critical Care software (GE Healthcare). Data were collected for all variables every 1–3 min.
Pre-processing of ABP
Incomplete data for most of the patients at day 3 (d3) precluded reliable analysis and only values for day 1 (d1) and day 2 (2) were therefore analyzed. Details of the ABP recordings and ABP signal pre-processing are presented in Supplementary Fig. 1. The signal pre-processing aimed to exclude extreme values that were obviously not due to pathophysiological processes but specifically due to technical artifacts (e.g., infusion, motion, or blood sampling from the arterial line), without excluding variations that could potentially contain meaningful information. However, time series of ABP measurements consist of observations from complex, non-stationary processes of non-normally distributed data with non-linear behavior, including abrupt changes, for which the traditional value of 1.5 times the interquartile range (IQR) cannot be applied to define outliers. We thus used a quantitative approach, including a heuristic determination of the threshold, followed by visual inspection. Aberrant values were considered to be any value >3 times the IQR from the 0.25 and 0.75 quantiles of the residuals of the linear fit of 5-h sliding windows and were removed. The quality of the artifact removal was subsequently visually checked for all patients. Next, data were aligned by postnatal age (hours and minutes since birth) and the median values of the MABP were calculated on runs of 5 min, precluding extremely short events that would influence the analysis. Third, daily minimum MABP thresholds over 5, 10, 20, 30, 40, 50, and 60 consecutive minutes were extracted for the first two days of life for each infant.
Brain MRI assessment and analysis
All MRI examinations were performed at TEA with a 1.5 T (Avanto, Siemens, Erlangen, Germany) or 3.0 T (Magnetom Prisma, Siemens, Erlangen, Germany) instrument using a standard 16- or 64-channel head coil, without sedation, during the post-feed period. The sequences used in the study for the two MR devices are described in the Supplementary Methods.
Imaging data were analyzed using Osirix MD v11 software (Geneva, Switzerland) by two independent assessors (R.B. and C.H.) and inter-observer disagreements were resolved under the supervision of a senior pediatric radiologist with 35 years of experience (S.H.). Both primary MRI assessors and the arbitrator were blinded to the ABP data and clinical characteristics of the subjects.
A standardized scoring system was used to evaluate cerebral WM, cortical and deep gray matter (GM), and cerebellum abnormalities, as reported by Kidokoro et al.11. The Kidokoro scoring system is described in Supplementary Table 1. The thinning of the corpus callosum was removed from the scoring system because it was considered difficult to assess in our brain MRI. The primary endpoint was the association of the ABP trend and variability with the occurrence of brain abnormality according to the Kidokoro score, calculated as the sum of the four regional subscores. Brain abnormality at TEA-MRI was defined as a Kidokoro score ≥1.
Statistical analysis
Patient characteristics are reported as numbers (with percentages) for categorical variables and as medians (IQR) for continuous variables. Categorical variables were compared using the chi-square or Fisher’s exact test and continuous variables using the Mann–Whitney U test, as appropriate.
To identify independent predictive variables of normal brain TEA-MRI, we fitted multivariate logistic regression models for both d1 and d2. Independent variables were selected for inclusion in the analyses through LASSO regression to avoid inflated Type I errors.12 Thus, the lowest daily MABP for 5, 10, 20, 30, 40, 50, and 60 consecutive minutes and clinically relevant variables, including gestational age at birth, birthweight, sex, and the need for hemodynamic support, were included as covariates. This procedure allowed selecting only one duration of the lowest daily mean ABP for both d1 and d2, which were used in subsequent analyses.
The performance of the daily lowest MABP and that of the best model selected through LASSO regularization were evaluated by ROC curve analysis. The test is considered to be poorly accurate for an area under the curve (AUC) ≤ 0.7, moderately accurate for 0.7 < AUC ≤ 0.9, and highly accurate for 0.9 < AUC ≤ 1. The best cut-off value was defined as the one that maximized the difference between true positive subjects and false positives following the Youden index method.
Sensitivity analyses were performed to account for the effect of incomplete datasets (i.e., infants with less than 24 h of invasive blood pressure recording on each day) on sensitivity, specificity, and accuracy. We performed the same ROC analysis for the entire population and the subpopulation of patients with least 22 h of recording, i.e., 66 patients on d1 and 82 patients on d2. Another sensitivity analysis explored the hypothesis that cumulative, instead of consecutive, episodes of low MABP may be differently associated with normal TEA-MRI. We repeated the same analyses including the daily threshold MABP with an increasing number of 1 to 8 cumulative 5-min episodes of low ABP, whether they were contiguous or not. All statistical analyses were performed and graphics generated using R software (Vienna, Austria. https://www.R-project.org/). P values < 0.05 were considered significant.
Results
The flow chart of the study is shown in Fig. 1 and the baseline characteristics of infants with (n = 151) and without (n = 73) umbilical artery catheterization at birth are presented in Supplementary Table 2. We finally analyzed TEA-MRI and ABP monitoring data of 99 very premature babies (median [IQR] gestational age: 26.3 [25.1–27.5] weeks) assessed by the Kidokoro score on MRI performed at 40.3 [40.0–41.1]) weeks. Overall, the median [IQR] Kidokoro score was 2 [1–4] and 78/99 (79%) infants had an abnormal MRI. The distribution of the Kidokoro score according to gestational age at birth is presented in Supplementary Fig. 2. Aside from birthweight and gestational age at birth, co-morbidities significantly associated with evidence of brain injury (Kidokoro score ≥1) were fetal growth restriction (p = 0.01), mean duration of non-invasive ventilation (p = 0.01), late-onset sepsis (p = 0.01), and bronchopulmonary dysplasia as physiologically defined at 36 weeks of postmenstrual age (p = 0.003) (Table 1). Volume expansion and inotrope exposure are also presented in Table 1.
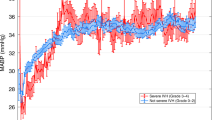
The mean, systolic, and diastolic ABP increased significantly with gestational age and birthweight. The trends in MABP values recorded during the first 72 postnatal hours according to gestational age at birth (per week) and birthweight (per z-score categorized in tertile) are presented in Fig. 2. The LASSO procedure selected 30 consecutive minutes on d1 and 10 consecutive minutes on d2 as the most relevant durations of lowest MABP to be associated with brain TEA-MRI findings (crude OR [95% CI], 1.11 [1.02–1.23], p = 0.03, and 1.13 [1.01–1.27], p = 0.03, respectively) (Models 1a and 1b in Fig. 3a, b). These OR values show the increasing chance of having normal TEA-MRI for each mmHg increase in the lowest MABP nadir recorded during a 30-min duration on d1 and 10-min duration on d2. ROC curve analysis showed optimal thresholds of 30.25 mmHg on d1 and 33.25 mmHg on d2 to predict the TEA-MRI (Fig. 3C). This significant association persisted after adjustment with covariates including birthweight, gestational age, sex, and inotrope exposure. The LASSO method selected the decile of the birthweight and the lowest MABP for 30 min on d1 and 10 min on d2 for inclusion in the final models (adjusted OR [95% CI], 1.16 [1.04–1.31], p = 0.011, and 1.19 [1.05–1.37], p = 0.008, respectively) (Models 2a and 2b in Fig. 3a, b). The areas under the ROC curve of Models 2a and 2b were 74% and 75%, respectively (Fig. 3c). In our population, gestational age at birth, sex, and inotrope exposure were not significantly associated with the TEA-MRI findings.
Panel a represents the association for d1 MABP recordings and panel b for d2 MABP recordings. The LASSO method selected the decile of the birthweight and the lowest MABP for 30 min on d1 and 10 min on d2 for inclusion in the final Models 2a and 2b. Panel c represents ROC curve analysis showing the performance of the daily lowest MABP levels alone (red line, Models 1a and 1b) to predict normal brain TEA-MRI and of the final models including both daily lowest MABP levels and the birthweight decile (blue line) on d1 (left) and d2 (right). Sensitivity (Se), specificity (Sp), and likelihood ratios (LR) refer to the optimal threshold determined for Models 1a and 1b following the Youden index method. MABP mean arterial blood pressure, OR odds ratio, CI confidence interval, AIC Akaike information criterion, AUC area under the receiving operating curve, Sp specificity, Se sensitivity, LR+ positive likelihood ratio, LR− negative likelihood ratio.
A sensitivity analysis showed similar results for patients with good-quality ABP monitoring available for more than 22 h per day (Supplementary Fig. 3). The specificity, sensitivity, and positive and negative likelihood ratios (LR+, LR−) of Models 1 (crude) and 2 (birthweight adjusted) are presented in Supplementary Table 3.
Another sensitivity analysis explored the association of cumulative, instead of consecutive, episodes of low MABP with TEA-MRI findings. A maximum of the four (on d1) and two (on d2) lowest MABP episodes of 5 min were significantly associated with normal TEA-MRI after adjustment for birthweight decile (adjusted OR [95% CI], 1.16 [1.03–1.32], p = 0.019, and 1.17 [1.04–1.35], p = 0.017, respectively).
Discussion
Our data show low MABP, as measured by continuous monitoring, to be associated with subsequent brain lesions detected by TEA-MRI in very preterm infants. Interestingly, these long-term MRI findings did not relate to early IVH, as our incidence of high-grade IVH was particularly low, but consistent with that of the SwissEpo trial.13 A 10-min period of MABP nadir on d2 was the best predictor to identify the patients with normal TEA-MRI. This relatively long mean ABP threshold needed to accurately predict the MRI findings several weeks after birth may be related to the relatively poor sampling of ABP data (one median value of mean ABP for each 5 min was finally analyzed). The MABP thresholds of 30.25 mmHg on d1 and 33.25 mmHg on d2 are consistent with a population estimate of blood pressure values previously published in infants <28 weeks of gestation.14
The distribution of the Kidokoro scores implies that this scoring system may not be sufficiently sensitive to detect mild injury in our cohort of very preterm infants, despite its ability to provide a structured and comprehensive evaluation of neonatal brain damage. Other TEA-MRI evaluation scales have been developed to grade the severity of brain damage and predict neurodevelopmental outcomes in preterm infants.15,16 These scales focus on cerebral WM and cortical GM and thus may underestimate other substantial damage in the basal ganglia or cerebellum. The Kidokoro score was therefore preferred in this study, although it does not consider more subtle damage to transient fetal compartments that persist to TEA, as recently reported.17
Several studies have reported greater variability in early continuous ABP recording in infants who develop high-grade IVH within the first postnatal week.6 Conversely, Bass et al.18 reported no significant differences for the lowest mean blood pressure during the first 7 days of life in very low-birthweight infants, with or without major brain lesions, whereas infants with parenchymal hemorrhage required more volume expansion and vasopressor support within the first two postnatal days. Interestingly, in the aforementioned study, infants with WM injury detected by ultrasound were significantly associated with maternal chorioamnionitis. Many studies have emphasized the close relationship between perinatal inflammation and brain vulnerability in very preterm infants, even when severe brain lesions are not detectable.19,20,21 Although further studies are needed to elucidate the effects of chorioamnionitis or fetal inflammatory response syndrome on transitional hemodynamic changes in preterm infants, certain evidence supports a role for perinatal inflammation in circulatory disturbances early after birth.22 In contrast to the established link between short-term IVH and fluctuations in pH, paO2, and paCO2, with subsequent cerebral blood flow instability, the exact relationship between perinatal inflammation and long-term abnormal brain maturation is still not fully understood. Here, only one infant was recorded with high-grade IVH and none had detectable seizures. Therefore, we can only speculate that low cerebral blood flow of the developing WM and defective brain autoregulation during the critical window of early stabilization after birth may play a key role to explain this association.23 In addition, such an association between the MABP and TEA-MRI is remarkable because it was observed after numerous postnatal complications that can occur between the first postnatal week and TEA. Several studies have also demonstrated the effect of postnatal inflammatory complications (late-onset sepsis, prolonged mechanical ventilation, bronchopulmonary dysplasia, necrotizing enterocolitis) in the development of brain lesions in preterm infants24,25 through the disruption of critical developmental processes in the developing brain, including that of differentiation of the oligodendroglial lineage and synaptogenesis.26,27
The strengths of this study include the prospective collection of data from a monocentric cohort with standardized care management, including management of low MABP. Second, continuous ABP recordings were collected from an arterial line placed into the descending aorta, allowing measurement of the true pressure, controlled by a standardized zero before recording.
This study also had several limitations. First, although we report an association between ABP fluctuations and the Kidokoro score at TEA, our study does not provide evidence that early ABP is more important than other postnatal variables in predicting brain damage or in selecting infants who should be assessed for brain imaging. Second, two MRI devices (1.5 and 3 T) were used to assess brain lesions. However, both are able to assess all items included in the Kidokoro score, and all MRI scans were centrally reviewed by two independent assessors on the same viewer system. ABP measurements were collected every 1–3 min and pre-processing to correct the datasets for motion artifacts, blood sampling, or infusion and remove aberrant values or outliers further reduced data sampling. We can assume that the results would have been optimized with better data sampling. The Kidokoro score may not be as suitable as expected to reveal subtle injury patterns in the developing brain and several authors have suggested slightly modified scoring systems, primarily published in 2013, to better describe extra-uterine brain development (myelination, gyration, ventricular dilation).28 Our study was not population-based and one of three very preterm infants admitted to the NICU did not undergo umbilical arterial catheterization, which may influence the outcome. Although there appears to be a selection bias between these two subpopulations, between-group differences may not be important, as many of the variables, including inotrope exposure, were not found to be confounders in the statistical analysis. Finally, the balance between insights from continuous ABP monitoring of vulnerable infants and the risk of central line-associated bloodstream infections should be carefully considered.
In conclusion, this study suggests that continuous ABP monitoring during the first two postnatal days may predict the presence of lesions detected on brain MRI at TEA in very premature neonates. Such early monitoring may help in assessing the clinical benefits of treatment for low ABP in truly hypotensive preterm infants. Indeed, despite some evidence that antihypotensive treatment may improve survival at hospital discharge without major morbidity for infants born before 29 weeks,29 the clinical benefits of such treatment are still debated.30 Thus, the MABP thresholds reported in this study should be considered with caution and interpreted according to local practices. Finally, the association between MABP and TEA-MRI findings revealed in this study provides some hint about mechanisms of brain injury in very preterm infants and may be informative for future clinical trials.
References
Pellicer, A., Valverde, E., Gaya, F., Quero, J. & Cabanas, F. Postnatal adaptation of brain circulation in preterm infants. Pediatr. Neurol. 24, 103–109 (2001).
Miall-Allen, V. M., de Vries, L. S. & Whitelaw, A. G. Mean arterial blood pressure and neonatal cerebral lesions. Arch. Dis. Child. 62, 1068–1069 (1987).
Watkins, A. M., West, C. R. & Cooke, R. W. Blood pressure and cerebral haemorrhage and ischaemia in very low birthweight infants. Early Hum. Dev. 19, 103–110 (1989).
Huvanandana, J. et al. Prediction of intraventricular haemorrhage in preterm infants using time series analysis of blood pressure and respiratory signals. Sci. Rep. 7, 46538 (2017).
Vesoulis, Z. A., Liao, S. M. & Mathur, A. M. Gestational age-dependent relationship between cerebral oxygen extraction and blood pressure. Pediatr. Res. 82, 934–939 (2017).
Vesoulis, Z. A. et al. Blood pressure extremes and severe IVH in preterm infants. Pediatr. Res. 87, 69–73 (2020).
Faust, K. et al. Short-term outcome of very-low-birthweight infants with arterial hypotension in the first 24 h of life. Arch. Dis. Child Fetal Neonatal Ed. 100, F388–F392 (2015).
Batton, B. et al. Early blood pressure, antihypotensive therapy and outcomes at 18-22 months’ corrected age in extremely preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 101, F201–F206 (2016).
Parodi, A. et al. Cranial ultrasound findings in preterm germinal matrix haemorrhage, sequelae and outcome. Pediatr. Res. 87, 13–24 (2020).
Volpe, J. J. The encephalopathy of prematurity–brain injury and impaired brain development inextricably intertwined. Semin. Pediatr. Neurol. 16, 167–178 (2009).
Kidokoro, H., Neil, J. J. & Inder, T. E. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am. J. Neuroradiol. 34, 2208–2214 (2013).
Belloni, A., Chernozhukov, V. & Hansen, C. Inference on treatment effects after selection amongst high-dimensional controls. Rev. Econ. Stud. 81, 608–650 (2014).
Natalucci, G. et al. Effect of early prophylactic high-dose recombinant human erythropoietin in very preterm infants on neurodevelopmental outcome at 2 years: a randomized clinical trial. JAMA 315, 2079–2085 (2016).
Vesoulis, Z. A., El Ters, N. M., Wallendorf, M. & Mathur, A. M. Empirical estimation of the normative blood pressure in infants <28 weeks gestation using a massive data approach. J. Perinatol. 36, 291–295 (2016).
Inder, T. E., Wells, S. J., Mogridge, N. B., Spencer, C. & Volpe, J. J. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J. Pediatr. 143, 171–179 (2003).
Miller, S. P. et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J. Pediatr. 147, 609–616 (2005).
Pittet, M. P., Vasung, L., Huppi, P. S. & Merlini, L. Newborns and preterm infants at term equivalent age: a semi-quantitative assessment of cerebral maturity. Neuroimage Clin. 24, 102014 (2019).
Bass, W. T. et al. Indices of hemodynamic and respiratory functions in premature infants at risk for the development of cerebral white matter injury. J. Perinatol. 22, 64–71 (2002).
Giraud, A. et al. Perinatal inflammation is associated with social and motor impairments in preterm children without severe neonatal brain injury. Eur. J. Paediatr. Neurol. 28, 126–132 (2020).
Dammann, O. & Leviton, A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr. Res. 42, 1–8 (1997).
Hagberg, H. & Mallard, C. Effect of inflammation on central nervous system development and vulnerability. Curr. Opin. Neurol. 18, 117–123 (2005).
Furukawa, S., Sameshima, H. & Ikenoue, T. Circulatory disturbances during the first postnatal 24h in extremely premature infants 25 weeks or less of gestation with histological fetal inflammation. J. Obstet. Gynaecol. Res. 34, 27–33 (2008).
Vesoulis, Z. A. & Mathur, A. M. Cerebral autoregulation, brain injury, and the transitioning premature infant. Front. Pediatr. 5, 64 (2017).
Adams-Chapman, I. Long-term impact of infection on the preterm neonate. Semin. Perinatol. 36, 462–470 (2012).
Patra, A., Huang, H., Bauer, J. A. & Giannone, P. J. Neurological consequences of systemic inflammation in the premature neonate. Neural Regen. Res. 12, 890–896 (2017).
Prasad, J. D. et al. Coordinated microstructural disruptions of the developing neocortex and subcortical white matter after prolonged mild-to-moderate early postnatal systemic inflammation. Brain Behav Immun 94, 338–356 (2021).
Volpe, J. J. Systemic inflammation, oligodendroglial maturation, and the encephalopathy of prematurity. Ann. Neurol. 70, 525–529 (2011).
Huning, B. et al. Relationship between brain function (AEEG) and brain structure (MRI) and their predictive value for neurodevelopmental outcome of preterm infants. Eur. J. Pediatr. 177, 1181–1189 (2018).
Durrmeyer, X. et al. Abstention or intervention for isolated hypotension in the first 3 days of life in extremely preterm infants: association with short-term outcomes in the Epipage 2 Cohort Study. Arch. Dis. Child Fetal Neonatal Ed. 102, 490–496 (2017).
Dempsey, E. M. et al. Hypotension in preterm infants (HIP) randomised trial. Arch. Dis. Child Fetal Neonatal Ed. 106, 398–403 (2021).
Funding
This work was funded by Department of Pediatrics, University Hospitals, Geneva.
Author information
Authors and Affiliations
Contributions
Conception and design, acquisition of data, or analysis and interpretation of data: R.B., C.H., A.H., F.B.-M., S.H., A.B., and O.B. Drafting of the article or critical revision for important intellectual content: R.B., A.B., and O.B. Final approval of the version to be published: all.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
A form providing written consent was collected from the parents of all patients at admission and the study was approved by the Swiss National Ethics Committee (BASEC No. 2018-00738).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Butticci, R., Habre, C., Hernandez, A. et al. Early arterial pressure monitoring and term-equivalent age MRI findings in very preterm infants. Pediatr Res 92, 822–828 (2022). https://doi.org/10.1038/s41390-021-01839-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01839-2