Abstract
Background
Low birth size (BS) and obesity have been associated with higher dehydroepiandrosterone sulfate (DHEAS) levels in childhood, insulin acting as a mediator, despite contradictory findings. To further explore these issues, we studied the associations between DHEAS, BS, adiposity, maternal characteristics, and cardiometabolic risk indicators, in participants of Generation XXI, a population-based birth cohort.
Methods
A sample of 700 children (mean age 7.1 yr) was randomly selected. Data on maternal characteristics, BS, body mass index (BMI), waist-to-height ratio, body fat (dual-energy X-ray absorptiometry), insulin, lipid profile, and high-sensitivity C-reactive protein were analyzed in relation to DHEAS.
Results
DHEAS was negatively associated with BS and positively associated with all adiposity indicators, with no sex differences. DHEAS was positively associated with insulinemia independently of the child’s BS or BMI. No significant association was found between DHEAS, maternal characteristics, lipid profile, or high-sensitivity C-reactive protein. Including insulin in the model did not affect the association between BS and DHEAS but reduced the magnitude of the BMI effect by 24% for boys and 30% for girls.
Conclusion
Higher DHEAS levels at 7 years old were associated with lower BS and higher adiposity. DHEAS levels were positively associated with insulinemia independently of BS or BMI.
Impact
-
Low birth weight and obesity have been associated with higher dehydroepiandrosterone sulfate (DHEAS) levels in prepuberty. Insulin has been suggested as a mediator, despite previous studies failing to show an association between DHEAS and insulin levels.
-
In a randomly selected population of 700 7-year-old children from the Generation XXI birth cohort, higher DHEAS levels were associated with a lower birth size and higher adiposity, with no sex differences.
-
DHEAS was positively related to insulinemia independently of the child’s birth size or body mass index.
-
No association was found between DHEAS and other cardiometabolic risk factors.
Similar content being viewed by others
Introduction
Adrenarche is the maturational increase in adrenal androgen production, including dehydroepiandrosterone (DHEA) and its sulfate (DHEAS). It only occurs in humans and in higher primate species that have a long childhood preceding the advent of puberty. It is a sensitive period of neurobiological development, since adrenal androgens are involved in sexual maturation, fertility, metabolism, and central nervous system buildout.1,2
DHEAS is a widely used marker for adrenarche, while DHEA’s more potent androgenic conversion products contribute to the clinical signs of adrenarche (pubic and axillary hair, adult-type body odor, acne, and seborrhea).3,4 DHEAS secretion rises at around 6–8 years of age and the clinical signs of adrenarche are usually seen after 8 years in girls and 9 in boys.3,4 However, in some children, clinical signs of androgen action together with a rise in serum DHEAS are observed earlier, without breast or testicular enlargement, a condition called premature adrenarche (PA).5
The mechanisms underlying adrenarche’s regulation are not completely understood. Pre and postnatal factors have been associated with higher DHEAS levels in childhood,3,4 mainly low birth weight,6,7,8,9 especially if accompanied by rapid weight or length/height gain in the first years of life,6,10,11 and childhood obesity.12,13,14
Data on DHEAS levels in healthy children, using population-based samples, have been published, mostly describing Finnish,7,11,12,15 Chilean,10,13,16 and British6 populations, but there are some puzzling issues. First, insulin was suggested to mediate the link between birth weight, higher prepubertal DHEAS levels, and childhood obesity,17,18,19 but data on this association are contradictory.7,12,13 Secondly, although PA was associated with a high-risk cardiovascular profile in prepuberty in a specific group of Catalan girls with premature pubarche and low birth weight,20 no adverse cardiovascular risk factors, besides obesity, have been found in children with higher serum DHEAS but without premature pubarche.12
Therefore, we aimed to assess the associations between serum DHEAS and birth weight and length, adiposity, cardiovascular risk indicators, and maternal characteristics, in 7-year-old children from a well-characterized Portuguese birth cohort.
Methods
Population
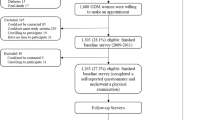
Participants of the present study are part of the birth cohort Generation XXI21 that recruited 8647 children born in 2005/2006 in all five public maternity units covering the metropolitan area of Porto, Portugal. At birth, 91.4% of the invited mothers agreed to participate. Data on demographic and socioeconomic characteristics, lifestyle, obstetric history, pre-pregnancy anthropometrics, and personal history of disease, were collected in a face-to-face interview, conducted by trained interviewers using structured questionnaires, during the hospital stay. Data on delivery and newborn characteristics (including gestational age, birth weight, and birth length) were additionally abstracted from clinical records.21,22 Birth weight and birth length z-scores were obtained according to the Fenton growth charts.23 For this study, children with a birth weight below the 10th percentile for sex and gestational age were categorized as small for gestational age (SGA), those between the 10th and the 90th percentile as appropriate for gestational age (AGA), and those above the 90th percentile as large for gestational age (LGA).23
In 2012/2014, the whole cohort was invited to the 7 years of age follow-up and 6889 (80% of the initial population) participated. For the current study, 700 children were randomly selected from those who have attended the 7-year-old follow-up, assuming 80% power and a 2-tail significance level of 0.05. Children included in the present study were similar to the remaining cohort regarding sex distribution, birth weight, height, and weight at 7 years old (data not shown). The study sample consisted of 351 girls and 349 boys with a mean age of 7.1 years (SD: 0.2).
Physical evaluation and body composition at 7 years old
Trained observers performed a physical examination and anthropometric measures. Participants were evaluated in underwear and bare feet. Weight was measured to the nearest 0.1 kg using a digital scale (Tanita®, Arlington Heights, IL), and standing height was measured to the nearest 0.1 cm using a wall stadiometer (Seca®, Hamburg, Germany). Body mass index (BMI) was calculated by dividing weight (kg) by squared height (m2). Weight, height, and BMI were transformed into age and sex-specific z-scores using World Health Organization (WHO) standards,24 and obesity was defined as a BMI z-score ≥ 2 standard deviations (SD) and overweight as a BMI z-score between 1 and 2 SD.
Waist circumference measurements were taken with a tape measure to the nearest 0.1 cm, at the umbilicus level, at the end of a normal expiration, with the child in a standing position, arms at the sides and feet together. Hip circumference was measured around the largest part of the hips. Central obesity was defined as waist circumference above the 90th percentile for sex and age, according to Fernandez’s reference data for European-American children.25 Waist-to-height ratio was calculated as an additional measure of body fat distribution.
Total body mass, fat body mass, fat mass percentage, lean body mass, trunk fat, and trunk mass were measured with the bladder emptied and lying in underwear by a dual-energy X-ray absorptiometry (DXA) scan in a Hologic Discovery QDR 4500W device (Hologic Inc., Bedford, Massachusetts).
At the 7-year-old visit, maternal height and weight were also measured. Maternal age at menarche was self-reported. Maternal pre-pregnancy weight and weight gain during pregnancy were abstracted from the pregnancy health card at recruitment.
Hormone and metabolic indicators at 7 years old
An overnight fasting venous blood sample was obtained before 11:00 a.m., after applying topical analgesic with lidocaine/prilocaine (EMLA cream). Serum was used for the biochemical measurements.
DHEAS and insulin were measured by electrochemiluminescence immunoassays on the Cobas® E411 (Roche); the limits of detection were 0.1 μg/dL and 0.2 μU/mL, respectively, and the intra- and interassay coefficients of variation were <2.3% and <3.2%, for insulin, and <2.5% and <3.9%, for DHEAS, respectively.
HbA1c was determined by high-performance liquid ion-exchange chromatography (BIO RAD VARIANT II), serum glucose was measured using a UV enzymatic assay (hexokinase method), and total-cholesterol, HDL-cholesterol, and triglycerides were measured using conventional assays, all on a Beckman-Coulter® AU5400 automated clinical chemistry analyzer. LDL-cholesterol was calculated using the Friedewald formula.26 High-sensitivity C-reactive protein (hsCRP) was measured using particle-enhanced immunonephelometric assay on a BN® II laser nephelometer. The Homeostasis model assessment for insulin resistance (HOMA-IR) was computed as “glucose (mg/dL) × insulin (mU/mL)/405”.27
Analyses were performed in the Clinical Pathology Department of Centro Hospitalar São João, Porto, Portugal.
Statistical analysis
Statistical analysis was performed using SPSS® (v.24; SPSS, IBM Corp., Armonk, NY).
Categorical and continuous variables are presented as counts (proportions), mean (SD), and median (range), as appropriate. Continuous variables were compared using the independent-samples Student t-test or the Mann–Whitney U test. Categorical variables were compared using Pearson’s chi-square test.
Differences in DHEAS levels among SGA, AGA, and LGA individuals and among normal weight, overweight and obese children were analyzed by ANCOVA, and post hoc pairwise comparisons among groups were conducted using Sidak correction.
The variation of DHEAS levels in relation to birth weight and length z-scores, adiposity indicators (weight, height, BMI, waist circumference, waist-to-height ratio, fat mass percentage, total body fat, trunk fat), biochemical indicators (fasting glucose, HOMA-IR, insulin, HbA1c, total-cholesterol, LDL and HDL-cholesterol, triglycerides and hsCRP) and maternal characteristics (maternal pre-pregnancy BMI, weight gain during pregnancy, or maternal age at menarche), were estimated fitting linear regression models, by sex strata. Variables with a distribution different from the normal were logarithmically transformed.
Logistic regression models were fitted to assess the association between the adiposity indicators and being at or above the 75th percentile for the DHEAS distribution of the studied population, arbitrarily defined as “high DHEAS levels”.
p-values < 0.05 were considered statistically significant.
Ethical issues
All the phases of the study complied with the Ethical Principles for Medical Research Involving Human Subjects expressed in the Declaration of Helsinki. The study was approved by the University of Porto Medical School/Centro Hospitalar São João ethics committee and parents or legal representatives of the children signed informed consent at the baseline and all the subsequent follow-up evaluations.
Results
The median serum DHEAS in the sample was 39.1 µg/dL (range: 1.8–237.2 µg/dL), 40.7 µg/dL (range: 2.7–231.4 µg/dL) in girls and 37.7 µg/dL (range: 1.8–237.2 µg/dL) in boys (p = 0.077) (Table 1).
Adiposity, metabolic and hormonal characteristics of the participants are detailed in Table 2. The prevalence of overweight was 23% and 15% were obese. Girls presented a higher proportion of central adiposity and a higher mean fat mass percentage. Girls had higher insulinemia, HOMA-IR, total-cholesterol, LDL-cholesterol, and higher triglyceride levels. No sex differences were found in birth weight and length z-scores nor maternal characteristics.
There was a significant difference in serum DHEAS levels among SGA, AGA and LGA children. The post hoc test (Sidak correction) showed higher DHEAS levels in SGA and lower DHEAS levels in LGA, when compared with each other and with AGA children (Fig. 1a). High DHEAS levels (>75th percentile of the whole study group) were found in 41% (57% females) of the SGA individuals, 23% (52% females) of the AGA individuals and 23% (44% females) of the LGA individuals.
**ANCOVA; the post hoc test (Sidak correction) p < 0.05 between normal weight and overweight and between normal weight and obese; p < 0.05 between overweight and obese. SGA: birth weight < 10th percentile; AGA: birth weight between the 10th and the 90th percentile; LGA: birth weight ≥ 90th percentile. Birth weight percentiles based on Fenton growth charts.23 Normal weight (BMI < 1 z-score); overweight (1 z-score ≤ BMI < 2 z-score); obese (BMI ≥ 2 z-score); p < 0.001. BMI z-scores based on WHO 2007.24
A significant difference in serum DHEAS levels was also seen among normal weight, overweight and obese children. The post hoc test (Sidak correction) showed higher DHEAS levels in obese and overweight children when compared with normal-weight children; obese children also presented significantly higher DHEAS than overweight children (Fig. 1b).
DHEAS levels were inversely related to birth weight and birth length (Table 3). DHEAS was positively associated with all the adiposity indicators: BMI, waist-to-height ratio, total body fat, fat mass percentage, and trunk fat. DHEAS was also positively associated with glucose, insulin, and HOMA-IR (Table 3). Associations were similar in magnitude and direction in both sexes.
No significant associations were found between DHEAS levels and total, LDL or HDL-cholesterol, triglycerides, hsCRP, maternal pre-pregnancy BMI, weight gain during pregnancy, nor maternal age at menarche (Table 3).
A positive association was found between insulin and BMI z-score at 7 years of age (β = 0.265 [95% CI: 0.231; 0.299]). No association was found between birth weight z-score and insulin (β = 0.027 [95% CI: −0.029; 0.083]) (data not shown).
In multivariable linear regression models, both lower birth weight, and higher BMI (model 1), and lower birth weight, higher BMI, and higher insulin (model 2) were associated with higher serum DHEAS (Table 4). Children with higher fasting insulin had higher DHEAS levels, even after adjustment for birth weight (or birth length) and BMI. A one percent increase in insulin levels increased DHEAS by 0.195%; for each 1 z-score increase in birth weight, DHEAS decreased by 15.4% and for each 1 z-score increase in BMI, DHEAS increased 13.4%. Similar results were found when total body fat rather than BMI was considered in the adjusted model (model 3). Also, the inclusion of birth length, instead of birth weight, did not change appreciably the strength and the direction of the associations (model 4).
Including insulin in the model did not affect the magnitude of the association between birth weight and DHEAS but reduced the magnitude of the BMI effect by 24% for boys and 30% for girls. Despite the reduction in the point estimate, the regression coefficients for BMI remained statistically significant.
The overall and sex-specific associations of adiposity with DHEAS levels above the 75th percentile are shown in Table 5. Obese children (odds ratio (OR): 2.4; 95% CI: 1.5–3.7) and those born SGA (OR 2.3; 95% CI: 1.4–3.7) had twice the odds of DHEAS above the 75th percentile. After adjustment for age, birth weight, and insulin (Table 5, model 3), obesity remained significantly associated with a DHEAS above the 75th percentile (OR 1.6 [95% CI: 1.0–1.6]).
Discussion
In our sample of 700 Portuguese children, we highlight two main results: DHEAS concentrations at age 7 were associated with lower birth size, higher adiposity, and higher insulinemia, independently of the child’s BMI or birth size, and insulin seemed to explain a part of the effect of BMI on DHEAS levels.
The median DHEAS levels in our population were 40.7 µg/dL in girls and 37.7 µg/dL in boys, slightly higher than those found in 7-year-old Chilean and Finnish children.12,13 The median DHEAS levels in a sample of 97 Portuguese 7-year-old PA patients (with clinical and biochemical criteria) from the same geographical area were 102 µg/dL in girls and 114 µg/dL in boys.28 Thus, we add to the existing knowledge by presenting the distribution of serum DHEAS levels in a population-based sample of Portuguese children.
DHEAS reflects adrenarche, although it is an inactive androgen metabolite and its levels do not always match the clinical signs of adrenarche.29 DHEAS cannot bind to the androgen receptor and cannot enter most cells as it needs active transport mechanisms as a hydrophilic steroid compound. The active androgens, such as testosterone and dihydrotestosterone, derive from DHEA, which is rarely (re-) generated from peripheral DHEAS.4,30,31 The activation of the androgen receptor is necessary for the appearance of adrenarche’s clinical signs, like pubic and axillary hair. Therefore, the phenotypic changes of pubarche depend on the concentrations of DHEAS, which reflects adrenal androgen output, the rate of DHEA peripheral conversion to biologically active androgens, and the androgen receptor activity.29,32
Adiposity increases adrenal androgen production and conversion to more potent androgens in peripheral fat tissue,15,32 thus explaining the association of prepubertal overweight and obesity with PA.33,34,35,36
DHEAS levels were positively associated with BMI, fat mass percentage, waist circumference, waist-to-height ratio, total body fat, and trunk fat. Furthermore, DHEAS levels were higher among obese and overweight prepubertal children, in comparison with their lean counterparts, and obese children had twice the odds of higher DHEAS levels (≥75th percentile). These results were in accordance with previous reports.12,13,14,15,37
Previous studies in the Finnish and the Chilean population found no association between DHEAS and insulinemia12,13 but we observed a linear association between serum DHEAS and fasting insulin levels and HOMA-IR. These associations remained after adjustment for birth size (both birth weight and length) and adiposity (both BMI and total body fat) and were similar in both sexes. Our results were consistent with case–control studies showing that PA prepubertal children have higher serum insulin17,36,38,39,40,41 and insulin resistance,39,40,42 independently of weight,36,43 and that insulin seems directly related to the degree of androgen excess.44,45,46,47
In our study, the point estimate of the association between BMI and DHEAS levels decreased 24% for boys and 30% for girls when insulin was included in the model. Also, the association between obesity and higher DHEAS levels decreased when insulin was included in the adjusted model (OR = 2.3 in the model adjusted for age and birth weight to OR = 1.6 in the model adjusted for age, birth weight, and insulin). These results suggest that insulin may mediate the effect of obesity on androgen production. This mediating role may be partly explained, as obesity increases insulin and insulin-like growth factors (IGFs)3,4,11 and the IGFs stimulate adrenal cell proliferation and steroidogenesis.48
We confirmed that DHEAS was inversely associated with birth size,6,7,8,9,11,12 with higher DHEAS levels being found in 7-year-old children with lower birth weight, independently of their BMI and insulinemia. We also showed that LGA children presented lower serum DHEAS than AGA and SGA children, as previously reported in the Finnish population.7 Metabolic programming during fetal and early postnatal life after intrauterine growth restriction can modulate insulin secretion,49 and hyperinsulinemia could justify the higher levels of DHEAS in SGA children. However, in our sample, the association between birth size and DHEAS remained similar after adjustment for insulin. We can hypothesize a role for neonatal stress and higher activation of the adrenal axis from prenatal life on, since there is some evidence of increased cortisol secretion and adrenal responsiveness to ACTH stimulation in SGA individuals.50
In our population, no significant associations between serum DHEAS and lipid profile or hsCRP were observed. Increased cardiometabolic risk and later development of the metabolic syndrome and ovarian hyperandrogenism were shown in a specific group of PA girls, who presented premature pubarche and overweight/obesity, and/or excessive weight gain in the first years of life, and/or low birth weight, and/or markedly elevated androgen levels in prepuberty.20,51,52 The association between DHEAS levels and cardiometabolic risk factors was explored in the Finnish population, and no association between higher DHEAS and an unfavorable metabolic profile, besides obesity, was found in children without premature pubarche; on the contrary, higher DHEAS levels were associated with a more favorable lipid profile, possibly due to their higher skeletal muscle mass.12
PA is more frequently observed in girls,3,4 but no sex difference in DHEAS levels was found in prepuberty.12,13 The large difference in the prevalence of PA between girls and boys, despite similar DHEAS levels in prepuberty, can be explained by a more efficient peripheral adrenal androgen conversion in girls not only to more potent androgens (due to their higher fat mass percentage, also seen in the present study), but also to estrogens through aromatase.15,16 Furthermore, girls are more predisposed to show signs of androgen action as their pilosebaceous unit is more sensitive to androgens. Hence, and even though DHEAS in our sample was similar in boys and girls, we decided to stratify our analysis by sex.
A major strength of our study is that we evaluated a large population-based sample at the same age. As serum DHEAS varies with age, we could analyze the association between birth weight, adiposity, and hormonal mediators, assessing the potential sex differences independently of such effect. We also had detailed information regarding body composition based on anthropometry measurements and total body DXA.
As potential limitations, we did not have information regarding Tanner stages nor other clinical signs of adrenarche, such as apocrine body odor, acne, or seborrhea, in this population. However, as we have analyzed a biochemical marker (serum DHEAS) and not the clinical signs of adrenarche, we do not expect this to affect our conclusions. DHEAS was measured with an immunoassay and not with liquid chromatography-tandem mass spectrometry; nevertheless, DHEAS measurement does not usually require mass spectrometric analytics, because its concentrations are sufficiently high to be analyzed reliably by immunoassays.
Although DHEAS has been traditionally considered the most relevant biomarker of adrenal androgen production, recent studies suggest that 11-oxygenated C19 adrenal-derived steroids are the main bioactive androgens during adrenarche and PA, and might be responsible for the clinical signs of adrenarche.53,54,55 However, large cross-sectional and longitudinal studies are needed.
In summary, we found that DHEAS levels in 7-year-old children were inversely associated with birth size and directly related to BMI, waist circumference, waist-to-height ratio, total body fat, and trunk fat. We also found that DHEAS levels were positively associated with insulinemia and HOMA-IR independently of birth size or BMI. We found no associations between DHEAS levels and lipid profile or hsCRP.
SGA, overweight, and obese children in prepubertal years may present with increased adrenocortical function. Whether higher androgen levels in these children will represent a higher risk for metabolic complications in the future deserves further investigation. As we continue to follow these children, our cohort may contribute to better explore these issues.
References
Prough, R. A., Clark, B. J. & Klinge, C. M. Novel mechanisms for DHEA action. J. Mol. Endocrinol. 56, R139–R155 (2016).
Ibanez, L., Dimartino-Nardi, J., Potau, N. & Saenger, P. Premature adrenarche–normal variant or forerunner of adult disease? Endocr. Rev. 21, 671–696 (2000).
Voutilainen, R. & Jaaskelainen, J. Premature adrenarche: etiology, clinical findings, and consequences. J. Steroid Biochem. Mol. Biol. 145, 226–236 (2015).
Utriainen, P., Laakso, S., Liimatta, J., Jaaskelainen, J. & Voutilainen, R. Premature adrenarche—a common condition with variable presentation. Horm. Res. Paediatr. 83, 221–231 (2015).
Rosenfield, R. L. Clinical review: identifying children at risk for polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 92, 787–796 (2007).
Ong, K. K. et al. Opposing influences of prenatal and postnatal weight gain on adrenarche in normal boys and girls. J. Clin. Endocrinol. Metab. 89, 2647–2651 (2004).
Nordman, H., Voutilainen, R., Antikainen, L. & Jaaskelainen, J. Prepubertal children born large for gestational age have lower serum DHEAS concentrations than those with a lower birth weight. Pediatr. Res. 82, 285–289 (2017).
Tenhola, S. et al. Increased adrenocortical and adrenomedullary hormonal activity in 12-year-old children born small for gestational age. J. Pediatr. 141, 477–482 (2002).
Ibanez, L., Lopez-Bermejo, A., Diaz, M., Suarez, L. & de Zegher, F. Low-birth weight children develop lower sex hormone binding globulin and higher dehydroepiandrosterone sulfate levels and aggravate their visceral adiposity and hypoadiponectinemia between six and eight years of age. J. Clin. Endocrinol. Metab. 94, 3696–3699 (2009).
Mericq, V., Pereira, A., Uauy, R. & Corvalan, C. Early BMI gain and later height growth predicts higher DHEAS concentrations in 7-year-old Chilean children. Horm. Res Paediatr. 87, 15–22 (2017).
Liimatta, J. et al. Tracking of serum DHEAS concentrations from age 1 to 6 years: a prospective cohort study. J. Endocr. Soc. 4, bvaa012 (2020).
Mantyselka, A. et al. Associations of dehydroepiandrosterone sulfate with cardiometabolic risk factors in prepubertal children. J. Clin. Endocrinol. Metab. 103, 2592–2600 (2018).
Corvalan, C., Uauy, R. & Mericq, V. Obesity is positively associated with dehydroepiandrosterone sulfate concentrations at 7 y in Chilean children of normal birth weight. Am. J. Clin. Nutr. 97, 318–325 (2013).
Kulle, A. E., Reinehr, T., Simic-Schleicher, G., Hornig, N. C. & Holterhus, P. M. Determination of 17OHPreg and DHEAS by LC-MS/MS: impact of age, sex, pubertal stage, and BMI on the Δ5 steroid pathway. J. Clin. Endocrinol. Metab. 102, 232–241 (2017).
Mantyselka, A. et al. The presentation of Adrenarche is sexually dimorphic and modified by body adiposity. J. Clin. Endocrinol. Metab. 99, 3889–3894 (2014).
Pereira, A., Iñiguez, G., Corvalan, C. & Mericq, V. High DHEAS is associated with earlier pubertal events in girls but not in boys. J. Endocr. Soc. 1, 800–808 (2017).
Ibanez, L. et al. Hyperinsulinemia and decreased insulin-like growth factor-binding protein-1 are common features in prepubertal and pubertal girls with a history of premature pubarche. J. Clin. Endocrinol. Metab. 82, 2283–2288 (1997).
Guercio, G., Rivarola, M. A., Chaler, E., Maceiras, M. & Belgorosky, A. Relationship between the GH/IGF-I Axis, insulin sensitivity, and adrenal androgens in normal prepubertal and pubertal boys. J. Clin. Endocrinol. Metab. 87, 1162–1169 (2002).
Guercio, G., Rivarola, M. A., Chaler, E., Maceiras, M. & Belgorosky, A. Relationship between the growth hormone/insulin-like growth factor-I axis, insulin sensitivity, and adrenal androgens in normal prepubertal and pubertal girls. J. Clin. Endocrinol. Metab. 88, 1389–1393 (2003).
Ibanez, L. et al. Postpubertal outcome in girls diagnosed of premature pubarche during childhood: increased frequency of functional ovarian hyperandrogenism. J. Clin. Endocrinol. Metab. 76, 1599–1603 (1993).
Larsen, P. S. et al. Pregnancy and birth cohort resources in Europe: a large opportunity for aetiological child health research. Paediatr. Perinat. Epidemiol. 27, 393–414 (2013).
Alves, E., Correia, S., Barros, H. & Azevedo, A. Prevalence of self-reported cardiovascular risk factors in Portuguese women: a survey after delivery. Int J. Public Health 57, 837–847 (2012).
Fenton, T. R. & Kim, J. H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 13, 59 (2013).
de Onis, M. et al. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 85, 660–667 (2007).
Fernández, J. R. et al. Changes in pediatric waist circumference percentiles despite reported pediatric weight stabilization in the United States. Pediatr. Obes. 12, 347–355 (2017).
Friedewald, W. T., Levy, R. I. & Fredrickson, D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499–502 (1972).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Santos-Silva, R., Costa, C., Castro-Correia, C. & Fontoura, M. Clinical, biochemical and gender characteristics of 97 prepubertal children with premature adrenarche. J. Pediatr. Endocrinol. Metab. 32, 1247–1252 (2019).
Utriainen, P., Voutilainen, R. & Jaaskelainen, J. Continuum of phenotypes and sympathoadrenal function in premature adrenarche. Eur. J. Endocrinol. 160, 657–665 (2009).
Hammer, F. et al. No evidence for hepatic conversion of dehydroepiandrosterone (DHEA) sulfate to DHEA: in vivo and in vitro studies. J. Clin. Endocrinol. Metab. 90, 3600–3605 (2005).
Idkowiak, J. et al. Steroid sulfatase deficiency and androgen activation before and after puberty. J. Clin. Endocrinol. Metab. 101, 2545–2553 (2016).
Liimatta, J. et al. Serum androgen bioactivity is low in children with premature adrenarche. Pediatr. Res. 75, 645–650 (2014).
Diaz, A., Bhandari, S., Sison, C. & Vogiatzi, M. Characteristics of children with premature pubarche in the New York metropolitan area. Horm. Res. 70, 150–154 (2008).
Zukauskaite, S., Lasiene, D., Lasas, L., Urbonaite, B. & Hindmarsh, P. Onset of breast and pubic hair development in 1231 preadolescent Lithuanian schoolgirls. Arch. Dis. Child. 90, 932–936 (2005).
Cebeci, A. N. & Tas, A. Higher body fat and lower fat-free mass in girls with premature adrenarche. J. Clin. Res. Pediatr. Endocrinol. 7, 45–48 (2015).
Utriainen, P., Jääskeläinen, J., Romppanen, J. & Voutilainen, R. Childhood metabolic syndrome and its components in premature adrenarche. J. Clin. Endocrinol. Metab. 92, 4282–4285 (2007).
Genazzani, A. R., Pintor, C. & Corda, R. Plasma levels of gonadotropins, prolactin, thyroxine, and adrenal and gonadal steroids in obese prepubertal girls. J. Clin. Endocrinol. Metab. 47, 974–979 (1978).
Paterson, W. F. et al. Exaggerated adrenarche in a cohort of Scottish children: clinical features and biochemistry. Clin. Endocrinol. 72, 496–501 (2010).
Evliyaoglu, O., Berberoglu, M., Adiyaman, P., Aycan, Z. & Ocal, G. Evaluation of insulin resistance in Turkish girls with premature pubarche using the homeostasis assessment (HOMA) model. Turk. J. Pediatr. 49, 165–170 (2007).
Escobar, M. E., Gryngarten, M. G., Ballerini, M. G. & Ropelato, M. G. Clinical, hormonal and metabolic findings in argentinean girls with premature pubarche. Rev. Argent. Endocrinol. Metab. 44, 6–16 (2007).
Sagodi, L., Barkai, L., Tombacz, A. & Vamosi, I. Hyperinsulinemia, insulin-like growth factor-I, insulin-like growth factor-binding protein-1, and sex-hormone binding-globulin in prepubertal and pubertal girls with premature adrenarche. Orv. Hetil. 144, 67–72 (2003).
Akin, O. et al. Vitamin D insufficiency is related to premature adrenarche. Horm. Res. Paediatr. 86, 152–152 (2016).
Ibanez, L., Potau, N., Chacon, P., Pascual, C. & Carrascosa, A. Hyperinsulinaemia, dyslipaemia and cardiovascular risk in girls with a history of premature pubarche. Diabetologia 41, 1057–1063 (1998).
Ibanez, L. et al. Hyperinsulinemia in postpubertal girls with a history of premature pubarche and functional ovarian hyperandrogenism. J. Clin. Endocrinol. Metab. 81, 1237–1243 (1996).
Vuguin, P., Grinstein, G., Freeman, K., Saenger, P. & Nardi, J. D. Prediction models for insulin resistance in girls with premature adrenarche - The premature adrenarche insulin resistance score: PAIR score. Horm. Res. 65, 185–191 (2006).
DiMartino-Nardi, J. Pre- and postpubertal findings in premature adrenarche. J. Pediatr. Endocrinol. Metab. 13, 1265–1269 (2000).
Vuguin, P., Linder, B., Rosenfeld, R. G., Saenger, P. & DiMartino-Nardi, J. The roles of insulin sensitivity, insulin-like growth factor I (IGF-I), and IGF-binding protein-1 and -3 in the hyperandrogenism of African-American and Caribbean Hispanic girls with premature adrenarche. J. Clin. Endocrinol. Metab. 84, 2037–2042 (1999).
l’Allemand, D. et al. Insulin-like growth factors enhance steroidogenic enzyme and corticotropin receptor messenger ribonucleic acid levels and corticotropin steroidogenic responsiveness in cultured human adrenocortical cells. J. Clin. Endocrinol. Metab. 81, 3892–3897 (1996).
Hales, C. N. & Barker, D. J. The thrifty phenotype hypothesis. Br. Med Bull. 60, 5–20 (2001).
Ward, A. M. V., Syddall, H. E., Wood, P. J., Chrousos, G. P. & Phillips, D. I. W. Fetal programming of the Hypothalamic-Pituitary-Adrenal (HPA) axis: low birth weight and central HPA regulation. J. Clin. Endocrinol. Metab. 89, 1227–1233 (2004).
Ibanez, L., Potau, N., Francois, I. & de Zegher, F. Precocious pubarche, hyperinsulinism, and ovarian hyperandrogenism in girls: relation to reduced fetal growth. J. Clin. Endocrinol. Metab. 83, 3558–3562 (1998).
Likitmaskul, S. et al. ‘Exaggerated adrenarche’ in children presenting with premature adrenarche. Clin. Endocrinol. (Oxf.) 42, 265–272 (1995).
Rege, J. et al. 11-Ketotestosterone is the dominant circulating bioactive androgen during normal and premature adrenarche. J. Clin. Endocrinol. Metab. 103, 4589–4598 (2018).
Turcu, A. F. & Auchus, R. J. Clinical significance of 11-oxygenated androgens. Curr. Opin. Endocrinol. Diabetes Obes. 24, 252–259 (2017).
Wise-Oringer, B. K. et al. The unique role of 11-oxygenated C19 steroids in both premature adrenarche and premature pubarche. Horm. Res. Paediatr. 93, 460–469 (2020).
Acknowledgements
We gratefully acknowledge the families enrolled in Generation XXI for their kindness, all members of the research team for their enthusiasm and perseverance, as well as the participating hospitals and their staff for their help and support. Generation XXI was supported by the European Regional Development Fund (ERDF) through the Operational Programme Competitiveness and Internationalization and national funding from the Foundation for Science and Technology (FCT), Portuguese Ministry of Science, Technology and Higher Education under the project “HIneC: When do health inequalities start? Understanding the impact of childhood social adversity on health trajectories from birth to early adolescence” (POCI-01-0145-FEDER-029567; Reference PTDC/SAU-PUB/29567/2017). It is also supported by the Unidade de Investigação em Epidemiologia - Instituto de Saúde Pública da Universidade do Porto (EPIUnit) (UIDB/04750/2020), Administração Regional de Saúde Norte (Regional Department of Ministry of Health) and Fundação Calouste Gulbenkian. This study was also supported by a grant from the Portuguese Society of Paediatrics. A.C.S. is funded by FCT Investigator contracts IF/01060/2015.
Author information
Authors and Affiliations
Contributions
R.S.-S.: contributed to the design of the study and the acquisition of data, conducted the analysis, and interpretation of data, and drafted the article. M.F.: contributed to the conception of the study, the interpretation of data, and revised the article critically for relevant knowledgeable content. J.T.G.: conducted laboratory analysis and revised the article critically for relevant knowledgeable content. H.B.: assembled the cohort, contributed to the conception of the study, and revised the article critically for relevant knowledgeable content. A.C.S.: contributed to the conception of the study and the acquisition of data, participated in the analysis and interpretation of data, and revised the article critically for relevant knowledgeable content. All the authors have accepted responsibility for the entire content of this manuscript and approved submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patients’ consent
All the phases of the study complied with the Ethical Principles for Medical Research Involving Human Subjects expressed in the Declaration of Helsinki. The study was approved by the University of Porto Medical School/S. João Hospital Centre ethics committee and parents or legal representatives of the children signed informed consent at the baseline and all the subsequent follow-up evaluations. Children gave their oral assent in all phases of the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Santos-Silva, R., Fontoura, M., Guimarães, J.T. et al. Association of dehydroepiandrosterone sulfate, birth size, adiposity and cardiometabolic risk factors in 7-year-old children. Pediatr Res 91, 1897–1905 (2022). https://doi.org/10.1038/s41390-021-01706-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01706-0
This article is cited by
-
Birth size and the serum level of biological age markers in men
Scientific Reports (2023)
-
Association between dehydroepiandrosterone sulphate levels at 7 years old and bone mineral density at 10 years old: a prospective cohort study
European Journal of Pediatrics (2022)