Abstract
Background
Data on microstructural white matter integrity in preterm infants with post-hemorrhagic ventricular dilatation (PHVD) using diffusion tensor imaging (DTI) are limited. Also, to date, no study has focused on the DTI changes in extremely preterm (EP) infants with PHVD.
Methods
A case–control study of EP infants <28 weeks’ gestation with PHVD was conducted. Diffusivity and fractional anisotropy (FA) values of corticospinal tracts (CST) and corpus callosum (CC) were measured using DTI at term-equivalent age. Outcomes were assessed at 2-years-corrected age.
Results
Twenty-one infants with PHVD and 21 matched-controls were assessed. FA values in the CC were lower in infants with PHVD compared with controls (mean difference, 0.05 [95% confidence interval (CI), 0.02–0.08], p < 0.001). In infants with periventricular hemorrhagic infarction, FA values in the CC were lower than in controls (mean difference, 0.05 [95% CI, 0.02–0.09], p = 0.005). The composite cognitive and motor scores were associated with the FA value of the CC (coefficient 114, p = 0.01 and coefficient 147, p = 0.004; respectively).
Conclusions
Extremely preterm infants with PHVD showed lower FA values in CC. A positive correlation was also shown between the composite cognitive and motor scores and FA value of the CC at 2-years-corrected age.
Impact
-
Extremely preterm infants with post-hemorrhagic ventricular dilatation showed lower fractional anisotropy values in their corpus callosum compared with controls reflecting the impaired microstructure of these commissural nerve fibers that are adjacent to the dilated ventricles.
-
Impaired microstructure of the corpus callosum was shown to be associated with cognitive and motor scores at 2-years-corrected age.
Similar content being viewed by others
Introduction
Germinal matrix hemorrhage-intraventricular hemorrhage (GMH-IVH) is the most common neurological disorder of the very preterm population and the risk of an adverse neurodevelopmental outcome increases significantly when GMH-IVH is complicated by post-hemorrhagic ventricular dilatation (PHVD).1,2,3 Experimental models and human studies have consistently shown that the mechanisms of the detrimental effects of PHVD are multifactorial and mediated mainly by mechanical distortion, neuroinflammation, and neurotoxicity.1,4,5,6,7 Prolonged distending pressure of the ventricles, along with inflammatory substances and blood products in the hemorrhagic cerebrospinal fluid (CSF), can lead to secondary white matter injury and disturbed myelination as seen in infants with progressive PHVD and subsequent neurodevelopmental sequelae.3,8,9,10
The application of magnetic resonance imaging (MRI) combined with advanced analytical approaches is increasingly being used to enhance our understanding of the neurodevelopmental problems that preterm infants face.11 Diffusion tensor imaging (DTI) is an advanced MRI technique, which allows the examination of fiber tracts in the neonatal brain by utilizing the three-dimensional anisotropy of water diffusion.12,13 DTI is widely applied in studies of the developing brain, as measures obtained with this technique provide objective and predictable indices of white matter development and injury.1,13,14 These indices include the molecular diffusion rate of water (mean diffusivity, MD), the directional preference of diffusion (fractional anisotropy, FA), the diffusion rate along the main axis of diffusion (axial diffusivity, AD), and the rate of diffusion in the transverse direction (radial diffusivity, RD).14 Very preterm infants with brain injury show lower FA and AD, and higher RD and MD values compared with infants without brain injury using DTI across multiple white matter regions, especially in those anatomically close to the ventricles, such as corpus callosum (CC) and corticospinal tracts (CST).15 While DTI has been used to assess white matter tracts in preterm infants with GMH-IVH, changes in white matter microstructure using DTI in infants with PHVD have not been studied extensively. Also, to date, no study has focused on the DTI changes in extremely preterm (EP) infants with PHVD.14,15,16 In this study, we sought to assess the effects of PHVD on FA, AD, RD, and MD values in extremely preterm infants and hypothesized that PHVD may adversely impact the highly dynamic and vulnerable maturational processes in the white matter and neurodevelopmental outcomes at 2-years-corrected age.
Methods
Study population
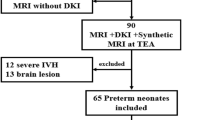
In this single-center retrospective case–control study, extremely preterm infants <28 weeks’ gestation with PHVD who were admitted to the level three neonatal intensive care unit of the Wilhelmina Children’s Hospital, University Medical Center Utrecht, between September 2007 and April 2016 were eligible. Sequential cranial ultrasonography (cUS) was performed by experienced attending neonatologists or physician assistants on admission, on days 3 and 7, and at least weekly thereafter until term-equivalent age (TEA). Scanning was conducted with a multi-frequency microconvex probe using a Toshiba Aplio Ultrasound System (Canon Medical Systems Inc., Tochigi, Japan). During the study period, the cUS scanning protocol remained unchanged. Infants with PHVD were included if they had progressive measurements of the ventricular index (VI) > 97th percentile and anterior horn width (AHW) > 6 mm on at least two cUS scans using reference charts described by Levene17 and Davies et al.,18 respectively. Ultrasonographic criteria and treatment thresholds for intervention are described in detail in a previous article.19 Periventricular hemorrhagic infarction (PVHI) was defined as a unilateral parenchymal hemorrhage ipsilateral to the GMH-IVH, as defined by Volpe.20 Infants were excluded if they had a chromosomal abnormality, genetic disorder, coexisting cerebellar hemorrhage, congenital or acquired central nervous system infection, and/or inborn errors of metabolism. For each infant with PHVD, a gestational age (GA) (within ±7 days) and sex-matched preterm infant without signs of hemorrhagic or ischemic preterm brain injury on cUS was included for the control group.
Demographic data were extracted from the patients’ files and/or electronic hospital database. Data on antenatal and perinatal factors including GA, birth weight, sex, the severity of GMH-IVH, and clinical data including the use of postnatal steroids, prolonged mechanical ventilation for >7 days, late-onset sepsis, necrotizing enterocolitis (NEC) requiring surgical treatment, hemodynamically significant patent ductus arteriosus and retinopathy of prematurity were collected. Due to the pseudonymization of clinically acquired data, written informed consent from the parents or legal guardians of the study infants was not deemed necessary by the Institutional Review Board (IRB), and a waiver of consent was provided for this study.
Neuroimaging protocol and DTI assessment
Term-equivalent age-MRI (TEA-MRI) was obtained using a 3-Tesla Philips Achieva MR scanner (Achieva, Philips Medical Systems, Best, the Netherlands). Prior to the MRI at TEA, infants were sedated, wrapped in a vacuum pillow, and positioned in an 8-channel SENSE head coil. Each neonate was given hearing protection consisting of Minimuffs (Natus Medical Incorporated, San Carlos, CA) and Earmuffs (EMs 4 Kids, Brisbane, QLD, Australia). DTI data were acquired in the axial plane with a slice thickness of 2 mm. The DTI sequences included a single non-diffusion-weighted image and additional diffusion-weighted images with a b-value of 800 s/mm2. Within the study period, the hospital scanning protocols changed; DTI scans with both 32 diffusion-weighted images (single-shot echo-planar imaging, repetition time 6817 ms, echo-time 97 ms, 50 × 2 mm slices, field of view 190 × 190 mm, matrix 128 × 128) and with 45 diffusion-weighted images (single-shot spin-echo echo-planar imaging sequence, repetition time 6500 ms, echo-time 80 ms, 45 × 2 mm slices, field of view 160 × 160 mm, matrix 80 × 80) were performed and used for this study. In the final analysis, FA, AD, RD, and MD were used to compare the groups.
Using the ExploreDTI toolbox (http://www.exploredti.com/) for Matlab (The MathWorks Inc., Natick, MA), scans were converted, corrected, and analyzed. Each scan was corrected for signal drift and subject motion. The DTI data were registered to the Oishi template with a rigid registration to correct for the different scan angulations of the subjects.21 The DTI tensor was estimated using the previously described REKINDLE (robust extraction of kurtosis indices with linear estimation) approach.22 DTI scans were then checked for quality using data quality summary and diffusion-weighted data were corrected for motion-induced outliers. Diffusion-weighted images with more than 10% outliers were removed, and if more than 10% of diffusion-weighted images had to be removed the DTI scan was excluded. Whole-brain tractography was performed with a seedpoint resolution of 1.5, seed fractional anisotropy threshold of 0.1, fiber length range of 30–500 mm, and angle threshold of 40°. Three fiber tracts were isolated; the CC and the bilateral CST. To isolate the fibers in the CC, AND-gates were manually drawn in the sagittal plane starting from the midsagittal line with two gates on either side. These gates define which fibers are included during tractography. For the CST, two gates were drawn in the axial plane: one in the anterior part of the middle cerebral peduncle where descending corticospinal and ascending sensorimotor fibers are separated from other descending fibers by the pontine crossing fibers, and one at the level where the CST and anterior limb of the internal capsule form the most well-defined angle as described previously.23 The left and right gates were placed on the same slide. Tracts that passed through both gates were selected for further analysis. In some cases, the selected tracts showed an abnormal course, e.g., crossing at the pontine level and returning to the contralateral motor cortex. We therefore only used the part of the tract between the two AND-gates for further analysis. This was done for both the CST and the CC. In a study of adult stroke patients, this approach was found to be more sensitive for differences between both CST and was found to have high inter-rater reliability (>90%).24 We, therefore, did not study an inter- or intra-rater reliability.
Neurodevelopmental assessment
Participants’ neurodevelopmental outcomes were assessed longitudinally as part of the standard neurodevelopmental follow-up program. Developmental assessments were performed by experienced developmental specialists (pediatric physiotherapist or pediatric psychologist) using the Bayley Scales of Infant and Toddler Development, Third Edition (BSITD-III), or Griffiths Mental Development Scales (GMDS). The composite cognitive and motor scores at 24-months-corrected age were categorized as the normal range (mean ± 1 standard deviation [SD]), subclinical range (<−1 SD), and clinical range (<−2 SD). Developmental Z-scores were calculated for cognitive and motor scores for each infant to compare different test types. Cerebral palsy (CP) and, if applicable, its type was defined based on the definition by Rosenbaum et al.25 The gross motor function classification system (GMFCS levels I–V) was used to grade the severity of CP.26 The presence of post-neonatal epilepsy requiring anti-seizure medication was retrieved from the infants’ files.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics, version 27 (IBM Corp., Armonk, N.Y.). Continuous variables were presented as mean (±SD) and median (interquartile range [IQR]) depending on their distribution. Categorical values were presented as number and percentage. The chi-squared and Fisher’s exact tests were used to compare categorical variables among groups. Mann–Whitney U test was used to compare non-parametric variables and Student t-test for comparison between variables with normal distribution. In infants with PHVD, subgroup analysis was done for infants with and without PVHI. The Kruskal–Wallis test and one-way analysis of variance (ANOVA) test were used to determine the difference in subgroup analysis. A post-hoc analysis was performed to determine the statistical differences in multi-group comparisons. Univariable linear regression included the entire cohort of 42 infants and this was used to determine risk factors for an adverse neurodevelopmental outcome. The sample size precluded multivariable regression analysis. Statistical significance was set at p < 0.05.
Results
Twenty-one infants with PHVD and 21 matched-controls were included in the analysis. The mean (±SD) gestational age was 26.5 ± 0.9 weeks for both groups. Birth weight was similar across the groups (960 ± 154 g vs 892 ± 180 g in infants with and without PHVD, respectively; p = 0.2). More infants required invasive mechanical ventilation in the PHVD group (63% vs 30%; p = 0.04). In infants with PHVD, 10 (48%) also had a unilateral PVHI. Clinical characteristics of included infants and distribution of the comorbidities are presented in Table 1.
Of the 21 infants with PHVD, 3 (14%) infants did not require intervention for PHVD; in these infants, PHVD resolved spontaneously under watchful monitoring. Eighteen (86%) infants required interventions, starting with lumbar punctures (LP). Out of these 18, fourteen (67%) underwent an early intervention and received LPs before the VI crossed the P97 + 4 mm line on cUS scans. Fifteen infants (71%) received a reservoir insertion following the LPs and eight (38%) required a ventriculo-peritoneal shunt for permanent CSF diversion.
DTI characteristics
There was a narrow range for performing the TEA-MRI and the mean ± SD postmenstrual age at the time of TEA-MRI was similar across the groups (38.8 ± 0.56 weeks and 38.8 ± 0.57 weeks in infants with PHVD and control infants, respectively; p = 0.8). The number of infants scanned with the updated protocol was the same across the groups (n = 12/21, 57% for both). In the PHVD group, 4 infants had diffusion-weighted scans with more than >10% motion-induced outliers. In these infants, a total of 10 DWI scans were removed. In the control group, 3 infants had scans with more >10% motion-induced outliers, resulting in the removal of 7 scans.
FA values in the CC were lower in infants with PHVD compared with controls (mean difference, 0.05 [95% confidence interval (CI), 0.02–0.08], p < 0.001). In infants with PHVD accompanied by PVHI, FA values in the CC were lower than in controls (mean difference, 0.05 [95% CI, 0.02–0.09], p = 0.005). FA values in the CC were similar between infants with PHVD and infants with PHVD accompanied by PVHI (p = 0.9). RD values in CC were higher in infants with PHVD compared with controls (mean difference, 91 × 10−6 mm2/s [95% CI, 3 × 10−6 to 179 × 10−6], p = 0.04). RD values in CC were also greater in infants with PHVD accompanied by PVHI than in controls (mean difference, 73 × 10−6 mm2/s [95% CI, 2 × 10−6–145 × 10−6], p = 0.004).
No significant differences were found in diffusivity and FA values in the CST between PHVD and control infants. In infants with PVHI, FA values of the CST ipsilateral to the PVHI showed a trend toward lower values when compared to those of controls (p = 0.09). Details of the DTI analysis for the CST and CC are presented in Tables 2 and 3.
Neurodevelopmental outcomes
All 42 infants were included in the outcome analysis. Of the infants with PHVD, 4 (19%) were assessed with GMDS and 17 (81%) with BSITD-III, while 5 infants (24%) were assessed with GMDS and 16 (76%) with BSITD-III in the control group (p = 0.7). The mean ± SD composite cognitive score was 92 ± 14 in infants with PHVD, and 102 ± 13 in the control group (p = 0.02); the composite motor score was 90 ± 13 and 105 ± 14, respectively (p < 0.001). Cognitive and motor Z-scores were greater in infants without PHVD (mean difference, 0.72 [95% CI, 0.12–1.3] and 1.1 [95% CI, 0.5–1.72]; p = 0.02 and <0.001, respectively) when compared with infants with PHVD.
Details of the neurodevelopmental outcomes in the subgroup analyses are presented in Table 4. In infants with PVHI, the mean ± SD FA values of the ipsilateral and contralateral CST were 0.32 ± 0.06 and 0.35 ± 0.06, respectively. Four (40%) infants with PVHI developed CP. In the univariate analysis, the FA value of the CC was associated with composite cognitive score (coefficient 114; 95% CI, 28–200; p = 0.01) and composite motor score (coefficient 147; 95% CI, 52–243; p = 0.004). The limited sample size (n = 42) precluded further multivariable regression analysis. The composite cognitive and motor outcome scores in relation to the FA values of the CC are presented in Fig. 1.
Discussion
In this single-center retrospective case–control study, we assessed the effects of PHVD on microstructural white matter microstructure with a specific focus on CC and CST in a cohort of EP infants. In keeping with our hypothesis, we found lower FA and higher RD values in CC in infants with PHVD compared to that of controls, reflecting impaired microstructure of these commissural nerve fibers located near the dilated ventricles. Infants with PHVD who also had PVHI, showed lower FA and higher RD values in CC compared to those of controls, although not different from those with PHVD only. Contrary to our hypothesis, we did not find altered diffusivity or FA values in CST in infants with PHVD; however, in infants with PVHI, ipsilateral FA values of CST showed a trend towards decrease reflecting the possible impact of parenchymal injury affecting the maturational processes in these white matter tracts. As expected, cognitive and motor scores were less favorable in infants with PHVD and FA values of the CC were associated with both cognitive and motor scores. To the best of our knowledge, this is the first report of impaired white matter microstructure demonstrated by DTI in an exclusive cohort of EP infants with PHVD.
DTI is the most widely used diffusion-weighted analysis approach in the developing brain, which has proven to be useful for assessing maturational characteristics.13 Prior work has shown that DTI is sensitive to the underlying microstructural neuroanatomical abnormalities in white matter associated with common forms of preterm brain injury.12,14,15,27 The developing preterm brain demonstrates a predictable pattern of DTI changes with decreasing MD and RD, and increasing FA values in the cerebral white matter.12,27,28 The increase in FA starts while the white matter structures are still at the pre-myelinating stage due to the escalation in axonal membrane maturation and change in axon caliber.13,29,30 At this early stage, the highest FA values are seen in the unmyelinated but well-organized commissural fibers of the CC.13 We focused on this stage of white matter maturation in our EP cohort and the impaired FA values in CC in the present study likely reflect the impact of injury in the context of PHVD. This observation is in line with previous observations of Lean et al.15 who showed altered DTI parameters in white matter regions anatomically close to the ventricles in very preterm infants.
The CST myelinates histologically between 32 and 35 weeks of gestation and this myelination is first seen on MRI of a very preterm infant in the posterior limb of the internal capsule from 36 to 38 weeks.31 The late stage of the increase in FA values in the developing white matter is closely related to the emergence of myelin, thus the initial signs are observed in the projection fibers of the CST around term.32 The lower FA in the CST in preterm infants with brain injury has been hypothesized to be indicative of fewer axons and less restricted water diffusion caused by impaired myelination alongside a propensity towards lower FA values due to immaturity.33 Lower FA values in CST in infants with PHVD compared to control infants in these studies may be due to local compression and impaired reabsorption of CSF, negatively affecting the DTI values due to proximity of this particular ROI to the enlarged ventricles. The CST has also been shown to be susceptible to inflammation, axonal injury leading to disturbances in myelin sheath development, and deformation caused by ipsilateral PVHI.15,23 The axonal injury following PVHI, so-called pre-Wallerian degeneration, begins within a week of the injury and progresses through several pathophysiologically distinct stages over the next weeks to evolve into Wallerian degeneration.34 Changes in water diffusion during the evolution of pre-Wallerian degeneration can be visualized early with DTI.23 We were unable to show impaired DTI values in CST in infants with isolated PHVD, which might be reflecting the protective effect of early intervention for PHVD in the majority of the infants studied. In a previous randomized controlled study, we found that almost twice as many infants undergoing high-threshold intervention (52% vs 27%) showed significant myelination delay in CST assessed with the Kidokoro score at TEA.35 This previous observation is in keeping with our current finding suggesting that timely interventions may prevent vulnerable regions of the developing brain from disturbances caused by PHVD. Avoidance of mechanical injury to the periventricular white matter with preservation of cerebral perfusion, and removal of inflammatory and neurotoxic substances from the CSF are among the several hypotheses why an earlier intervention may result in more favorable neurodevelopmental outcomes.36 We also observed a trend toward a decrease in ipsilateral FA values of CST in infants with PVHI; however, the limited number of infants in the present study, might have prevented this trend from reaching statistical significance. It is important to note that the small sample size might have also masked the DTI changes in CST in infants with isolated PHVD.
Microstructural white matter changes may explain the increased risk of adverse neurodevelopmental outcomes seen in preterm infants with brain injury.14 Roze et al.23 demonstrated that early neonatal DTI obtained within 4 weeks after birth is predictive of abnormal motor outcome in preterm infants with PVHI. By using an atlas-based approach, they showed that all infants with unilateral spastic CP had an FA asymmetry index of >0.05, which is the optimal cut-off value on early DTI. They suggested that early DTI could be used to predict a motor outcome, but the assessment of myelination at TEA may still be required.23 In another study using DTI, Lean et al.15 focused on white matter microstructure following different types of brain injury in very preterm infants and suggested that lower FA and higher MD values in CC were associated with increased risk for motor impairments at 2 years. More recently, Obeid et al.37 showed that in infants with high-grade GMH-IVH, increased frontal and temporal horn ratio correlated with lower FA in various white matter regions and higher GMFCS levels of CP. Similar impairments in cognitive and language development, and executive functioning were also reported by others following microstructural changes in CC as reflected by altered diffusivity at TEA.38,39 Our findings on cognitive and motor outcomes are in agreement with these previous observations, as we found a positive correlation between the FA value of the CC and composite cognitive and motor scores in the univariate analysis; however, the limited sample size precluded further multivariable regression in our model. Also in keeping with the recent literature, infants with PVHI had lower cognitive and motor scores compared to infants with isolated PHVD and controls.40
The present study has several limitations. First, the sample size was small, which limited the statistical analyses and precluded controlling for multiple factors in the regression model, including sex, age at MRI, and DTI sequence. However, infants were matched for gestational age and sex, and therefore it is unlikely that these factors have affected our results. Second, we were not able to take into account the different parts of the CC, but analyzed the DTI parameters of the CC as a single unit, thus we were unable to quantify the effect of injury on the genu, corpus, and splenium of the CC separately. Third, although the number of infants scanned with two different DTI protocols is the same across the groups, the study infants were not further matched for different DTI protocols. This may have caused variations in measured DTI parameters due to differences in image acquisition technique. Fourth, as reported by others, DTI fails to represent appropriately the tissue microstructure in the presence of crossing fibers, and in a restricted environment, diffusion of water molecules is no longer Gaussian and the tensor model deviates from the signal.13 Fifth, infants with PHVD were included in the present study if they had progressive measurements of the VI and AHW; however, thalamo-occipital distance could not be taken into account as this measurement was not available for all infants. The strength of this study is the selective recruitment of EP infants with GMH-IVH who developed PHVD while excluding infants with cerebellar injury, as this type of brain injury may also cause neurodevelopmental impairments in multiple domains.
In conclusion, in this case–control study, focusing on EP infants with PHVD, we showed lower FA values in the CC, reflecting the impaired microstructure of these commissural nerve fibers that are close to the dilated ventricles. We also found a positive correlation between FA values of the CC and composite cognitive and motor scores at 2-years-corrected age. Innovations in diffusion-weighted image acquisition are likely to enable neonatal white matter microstructure to be assessed in detail in the future.13 Further studies on a larger group of preterm infants with PHVD are needed to assess the effects of PHVD on white matter microstructure. These studies are warranted to further elucidate the effects of this common type of brain injury on fiber organization in different white matter tracts.
References
Ballabh, P. & de Vries, L. S. White matter injury in infants with intraventricular haemorrhage: mechanisms and therapies. Nat. Rev. Neurol. 17, 199–214 (2021).
Yeo, K. T. et al. Improving incidence trends of severe intraventricular haemorrhages in preterm infants <32 weeks gestation: a cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 105, 145–150 (2020).
El-Dib, M. et al. Management of post-hemorrhagic ventricular dilatation in the infant born preterm. J. Pediatr. https://doi.org/10.1016/j.jpeds.2020.07.079 (2020).
Strahle, J. M. et al. Role of hemoglobin and iron in hydrocephalus after neonatal intraventricular hemorrhage. Neurosurgery 75, 696–705 (2014).
Guo, J. et al. Minocycline-induced attenuation of iron overload and brain injury after experimental germinal matrix hemorrhage. Brain. Res. 1594, 115–124 (2015).
Del Bigio, M. R. Cellular damage and prevention in childhood hydrocephalus. Brain. Pathol. 14, 317–324 (2004).
Ulfig, N., Bohl, J., Neudorfer, F. & Rezaie, P. Brain macrophages and microglia in human fetal hydrocephalus. Brain. Dev. 26, 307–315 (2004).
Brouwer, M. J. et al. Effects of posthemorrhagic ventricular dilatation in the preterm infant on brain volumes and white matter diffusion variables at term-equivalent age. J. Pediatr. 168, 41–49 (2016).
Lockwood Estrin, G. et al. Altered white matter and cortical structure in neonates with antenatally diagnosed isolated ventriculomegaly. Neuroimage. Clin. 11, 139–148 (2016).
Ou, X. et al. Impaired white matter development in extremely low-birth-weight infants with previous brain hemorrhage. Am. J. Neuroradiol. 35, 1983–1989 (2014).
Kanel, D., Counsell, S. J. & Nosarti, C. Advances in functional and diffusion neuroimaging research into the long-term consequences of very preterm birth. J. Perinatol. 41, 689–706 (2021).
Miller, S. P. et al. Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. J. Magn. Reson. Imaging 16, 621–632 (2002).
Pecheva, D. et al. Recent advances in diffusion neuroimaging: applications in the developing preterm brain. F1000Res 7, 1326 (2018).
Dibble, M., Ang, J. Z., Mariga, L., Molloy, E. J. & Bokde, A. L. W. Diffusion tensor imaging in very preterm, moderate-late preterm and term-born neonates: a systematic review. J. Pediatr. 232, 48–58 (2021).
Lean, R. E. et al. Altered neonatal white and gray matter microstructure is associated with neurodevelopmental impairments in very preterm infants with high-grade brain injury. Pediatr. Res. 86, 365–374 (2019).
Morales, D. M. et al. Tract-specific relationships between cerebrospinal fluid biomarkers and periventricular white matter in posthemorrhagic hydrocephalus of prematurity. Neurosurgery 88, 698–706 (2021).
Levene, M. I. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch. Dis. Child. 56, 900–904 (1981).
Davies, M. W., Swaminathan, M., Chuang, S. L. & Betheras, F. R. Reference ranges for the linear dimensions of the intracranial ventricles in preterm neonates. Arch. Dis. Child. Fetal Neonatal Ed. 82, 218–223 (2000).
de Vries, L. S. et al. Treatment thresholds for intervention in posthaemorrhagic ventricular dilation: a randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 104, 70–75 (2019).
Volpe, J. J. Intraventricular hemorrhage in the premature infant-current concepts. Part II. Ann. Neurol. 25, 109–116 (1989).
Deshpande, R., Chang, L. & Oishi, K. Construction and application of human neonatal DTI atlases. Front. Neuroanat. 9, 138 (2015).
Tax, C. M., Otte, W. M., Viergever, M. A., Dijkhuizen, R. M. & Leemans, A. REKINDLE: robust extraction of kurtosis INDices with linear estimation. Magn. Reson. Med. 73, 794–808 (2015).
Roze, E. et al. Neonatal DTI early after birth predicts motor outcome in preterm infants with periventricular hemorrhagic infarction. Pediatr. Res. 78, 298–303 (2015).
Feldman, S. J., Boyd, L. A., Neva, J. L., Peters, S. & Hayward, K. S. Extraction of corticospinal tract microstructural properties in chronic stroke. J. Neurosci. Methods 301, 34–42 (2018).
Rosenbaum, P. et al. A report: the definition and classification of cerebral palsy April 2006. Dev. Med. Child. Neurol. Suppl. 109, 8–14 (2007).
Palisano, R. J. et al. Validation of a model of gross motor function for children with cerebral palsy. Phys. Ther. 80, 974–985 (2000).
de Bruine, F. T. et al. Tractography of developing white matter of the internal capsule and corpus callosum in very preterm infants. Eur. Radiol. 21, 538–547 (2011).
Kersbergen, K. J. et al. Microstructural brain development between 30 and 40 weeks corrected age in a longitudinal cohort of extremely preterm infants. Neuroimage 103, 214–224 (2014).
Wimberger, D. M. et al. Identification of “premyelination” by diffusion-weighted MRI. J. Comput. Assist. Tomogr. 19, 28–33 (1995).
Huppi, P. S. et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann. Neurol. 43, 224–235 (1998).
Cowan, F. M. & de Vries, L. S. The internal capsule in neonatal imaging. Semin. Fetal Neonatal Med. 10, 461–474 (2005).
Brody, B. A., Kinney, H. C., Kloman, A. S. & Gilles, F. H. Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J. Neuropathol. Exp. Neurol. 46, 283–301 (1987).
Rose, S. E. et al. Altered white matter diffusion anisotropy in normal and preterm infants at term-equivalent age. Magn. Reson. Med. 60, 761–767 (2008).
Aldskogius, H. & Kozlova, E. N. Central neuron-glial and glial-glial interactions following axon injury. Prog. Neurobiol. 55, 1–26 (1998).
Cizmeci, M. N. et al. Assessment of brain injury and brain volumes after posthemorrhagic ventricular dilatation: a nested substudy of the randomized controlled ELVIS trial. J. Pediatr. 208, 191–197 (2019).
Cizmeci, M. N., Groenendaal, F. & de Vries, L. S. Timing of intervention for posthemorrhagic ventricular dilatation: an ongoing debate. J. Pediatr. 234, 14–16 (2021).
Obeid, R. et al. The utility of the fronto-temporal horn ratio on cranial ultrasound in premature newborns: a ventriculomegaly marker. Pediatr. Res. 89, 1715–1723 (2021).
Thompson, D. K. et al. Regional white matter microstructure in very preterm infants: predictors and 7 year outcomes. Cortex 52, 60–74 (2014).
Thompson, D. K. et al. Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. Neuroimage 59, 3571–3581 (2012).
Cizmeci, M. N. et al. Randomized controlled early versus late ventricular intervention study in posthemorrhagic ventricular dilatation: outcome at 2 years. J. Pediatr. 1, S0022–S3476 (2020).
Acknowledgements
We thank the neonatal intensive care physicians, nurses, and physician assistants as well as developmental specialists and MR technicians for their dedicated help.
Author information
Authors and Affiliations
Contributions
A.N. conceptualized and designed the study, acquired the data including the chart review, and conducted the technical analysis of the brain MR images. She revised the article and approved the final version for publication. M.N.C. acquired the data, conducted the statistical analysis, wrote the first draft and revised version of the manuscript, and contacted the co-authors for further revisions. He approved the final version for publication. F.G. conducted the statistical analysis, critically reviewed, and approved the final version of the manuscript for publication. L.M.L. helped conceptualize and design the study, critically reviewed, and approved the final version of the manuscript for publication. C.K. helped to acquire the data, critically reviewed, and approved the final version of the manuscript for publication. M.J.N.L.B. helped acquiring the data, critically reviewed and approved the final version of the manuscript for publication. J.D. helped acquiring the data, critically reviewed and approved the final version of the manuscript for publication. L.S.d.V. conceptualized and designed the study, helped to draft the manuscript, and revising it for intellectual content. She approved the final version for publication. N.E.v.d.A. contributed significantly to the data acquisition by reviewing the brain imaging. He also helped to conceptualize the study and revising the manuscript for intellectual content. He approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent statement
Patient consent is not required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nieuwets, A., Cizmeci, M.N., Groenendaal, F. et al. Post-hemorrhagic ventricular dilatation affects white matter maturation in extremely preterm infants. Pediatr Res 92, 225–232 (2022). https://doi.org/10.1038/s41390-021-01704-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01704-2