Abstract
Background
Nonalcoholic fatty liver disease (NAFLD), a chronic liver disease in children, ranges from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH). We investigated the role of Angiopoietin-2 (Ang-2) as a biomarker for pediatric NAFLD-related liver damage.
Methods
We assessed the plasma levels of Ang-2 and cytokeratin-18 (CK18) fragments and their association with histologic activity in 76 children with NAFLD and 28 controls.
Results
The mean plasma levels of Ang-2 and CK18 were higher in children with NAFLD than in age-matched controls (Ang-2 155.4 ± 72.5 vs 7.5 ± 2.3 ng/mL, p < 0.001; CK18 390.4 ± 145.6 vs 193.9 ± 30.8 IU/L, p < 0.001). Ang-2 was significantly increased (p < 0.0001) in children with NASH (N = 41) while CK18 was significantly increased (p = 0.002) in children with fibrosis (N = 47). Ang-2 levels accurately predicted NASH (AUROC 0.911; 95% CI 0.844–0.979; p < 0.0001), while CK18 predicted both NASH (AUROC 0.827; 95% CI 0.735–0.919; p < 0.0001) and fibrosis (AUROC 0.724; 95% CI 0.611–0.837; p = 0.001). Ang-2 and CK18 in combination were good predictors of NASH with a sensitivity of 71.4% and a specificity of 100%.
Conclusions
In conclusion, our data suggested Ang-2 as a suitable biomarker of NASH in the pediatric population. However, our findings need external validation in other cohorts.
Impact
-
Several circulating factors have been extensively studied as potential biomarkers for NASH.
-
Angiopoietin-2 circulating levels are increased in children with NAFLD and are associated with NASH.
-
Angiopoietin-2 levels are more efficient than CK18 levels at assessing the most severe form of disease, and the combining of these two biomarkers reached a positive predictive value of 100% for NASH.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD) represents the most common cause of chronic liver disease and liver-related morbidity and mortality worldwide, both in adults and children.1,2 NAFLD encompasses nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH), with the latter including steatosis, liver lobular and portal inflammation, ballooning, and fibrosis.2 The resolution of NASH and fibrosis is the main focus of clinical trials. However, the chance of inflammation reversal is much higher than that of fibrosis reversal, and timely identification of inflammation seems to be crucial to reduce the burden of the liver disease in the youth. Therefore, it is pivotal to distinguish subjects with NAFL from those with NASH and fibrosis. In particular, in children, in whom fibrosis is less frequent than in adults, the identification of circulating markers has a great value to find patients with NASH that are more likely to progress to advanced fibrosis.1,2
The overall prevalence of NAFLD has reached approximately 10% in children and 17% in teenagers. It rises to 40–70% in children and adolescents with obesity. For this reason, the NAFLD is now considered one of the most frequent related-co-morbidity of obesity.3 NAFLD is associated with insulin resistance, type 2 diabetes, and other metabolic abnormalities, referring to metabolic syndrome.4 In light of this, NAFLD is pathogenically a multi-faced and complex disease mediated by several genetic, environmental, metabolic, and microbiological mechanisms, which are networked in the triggering of hepatic lipotoxicity and oxidative stress, and in systemic and liver inflammatory response.5
All of these mechanisms provide the starting point for the switch of the signaling molecules and pathways leading to hepatocellular damage, fibrosis, and cirrhosis, and eventually to distortion of the portal vein angioarchitecture.6 Among the multitude of risk factors and causes identified so far, the impairment of angiogenic process and the endothelial dysfunction emerge, as well as their interplay with the inflammatory response, as significant contributors to the development and progression of NAFLD-related liver damage.7,8 Hypoxia, chronic inflammation, fibrosis, and liver injury play key roles in stimulating vascular permeability and hepatic angiogenesis by inducing the release of pro-angiogenic factors, such as Angiopoietin-2 (Ang-2), from parenchymal and non-parenchymal cells.6,9 Ang-2 was found overexpressed in fibrotic livers, thus suggesting a role of this protein as a potential target to reduce both angiogenesis and hepatic inflammation during fibrogenesis.10 Lefere et al.9 recently investigated the role of Ang-2, in angiogenesis, leukocytes infiltration, and inflammation during NASH. The authors found that patients with NASH displayed elevated circulating levels of Ang-2 with respect to patient with simple steatosis or obese patients with diagnosis of NAFLD.9 Then, they reported that the high serum levels of Ang-2 in patients with NASH were correlated with grades of steatosis, lobular inflammation and ballooning, overall severity of liver histopathology, but not with fibrosis. This evidence encourages further investigations on the potential role of Ang-2 as a non-invasive circulating biomarker for NAFLD-related liver damage.
Hence, in the present study we sought to explore the association of the circulating levels of Ang-2 with the severity of the histological features in a sample of children and adolescents with biopsy-proven NAFLD, and its predictive accuracy alone or in combination with the levels of caspase-generated cytokeratin-18 (CK18) fragments, which is a widely recognized biomarker of pediatric NASH.11
Materials and methods
Study population
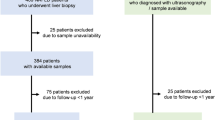
For this observational study we used biobanked blood samples collected from children evaluated at the Hepato-Metabolic Unit of the “Bambino Gesù” Children’s Hospital (Rome) between January 2012 and January 2014. All included children were Caucasians of Italian descent.
Children and adolescents were clinically evaluated for NAFLD following the diagnostic flow chart proposed by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Hepatology Committee.12 Accordingly, other liver diseases were ruled out. In fact, all children were tested for secondary causes of hepatic steatosis (i.e., viral liver disease, autoimmune hepatitis, Wilson’s disease, α-1-antitrypsin deficiency, endocrine, genetic and metabolic diseases, celiac disease, alcohol consumption, and use of drugs known to induce hepatic steatosis). In children with ultrasonographic evidence of severe hepatic steatosis diagnosis of NAFLD (N = 76) was confirmed by liver biopsy. Control group (N = 28) included children negative to steatosis by ultrasound and without other liver diseases.
Use of biobanked samples and associated anonymized data for future studies was approved by the local ethics committee (protocol number PNFI_OPBG_2010). Written informed consent for the use of the samples for future studies was obtained from the child’s parent at the time of the enrollment.
Anthropometric and biochemical parameters in children with NAFLD
Body weight, height, and waist circumference were measured with the patient wearing underwear according to a standard protocol. Body mass index (BMI) and BMI z-score (standard deviation score) were calculated with overweight and obesity status defined according to the Italian growth references.13,14
Venous blood samples were collected in the morning after an overnight fast of at least 8 h. Serum liver enzymes [aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase (GGT)], lipids [total cholesterol, high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol, and triglycerides], fasting glucose, and insulin levels were measured in all patients by using standard laboratory procedures at the main laboratory within the “Bambino Gesù” Children’s Hospital. Homeostasis model assessment score [HOMA-IR = (fasting insulin (mIU/L) × fasting glucose (mmol/L))/22.5)] was used for estimating insulin resistance; a cut-off value of >2.5 was considered as an index of insulin resistance.15
In children with NAFLD, whole-body insulin sensitivity was also computed on the basis of values of glucose and insulin during the oral test {ISI (insulin sensitivity index) = 10,000/√[fasting glucose (mmoL/L) × 18.182 × fasting insulin (μUI/mL)] × (mean glucose OGTT × 18.182 × mean insulin OGTT)}.16
Blood pressure was measured on the right arm using a standard sphygmomanometer; the average of three blood pressure values was reported. Elevated blood pressure was defined by systolic (SBP) or diastolic blood pressure (DBP) >95th percentile for age, height, and sex in patients below 13 years of age and as ≥120 mmHg in those older.17
Dyslipidemia was defined as high triglycerides (triglycerides ≥75 mg/dL in children aged below 9 years old and ≥90 mg/dL in those older); and/or high total cholesterol (total cholesterol ≥170 mg/dL); and/or low HDL-cholesterol (HDL-cholesterol ≤40 mg/dL); and/or high non-HDL cholesterol [(total cholesterol−HDL-cholesterol) ≥120 mg/dL].18
Prediabetes was defined as a fasting glucose ≥100 and <126 mg/dL (impaired fasting glucose, IFG) and/or 2 h plasma glucose ≥140 and <200 mg/dL following the standard oral glucose tolerance test performed with 75 g anhydrous glucose (impaired glucose tolerance, IGT).
Liver histology
Liver biopsies were performed using an automatic core biopsy 16- or 18-gauge needle under general anesthesia and ultrasound guidance. Hepatic steatosis, portal and lobular inflammation, hepatocyte ballooning, and fibrosis were features of NAFLD characterized at the histology. Diagnosis of NASH was performed by an expert pathologist (R.D.V.) and scored according to the scoring system developed by the National Institutes of Health-sponsored NASH Clinical Research Network.19 Briefly, hepatic steatosis was graded on a four‐point scale: 0 = steatosis involving fewer than 5% of hepatocytes, 1 = steatosis involving up to 33% of hepatocytes, 2 = steatosis involving 33–66% of hepatocytes, and 3 = steatosis involving more than 66% of hepatocytes. Lobular inflammation was graded on a four‐point scale: 0 = no foci, 1 = fewer than two foci per ×200 field, 2 = two to four foci per ×200 field, and 3 = more than four foci per ×200 field. Portal inflammation was graded on a four‐point scale: 0 = none, 1 = mild, 2 = moderate, and 3 = severe. Hepatocyte ballooning was graded from three-point scale: 0 = no balloon cells, 1 = few balloon cells and 2 = many/prominent balloon cells. The stage of hepatic fibrosis was quantified with a five‐point scale: 0 = no fibrosis, 1 = perisinusoidal or periportal fibrosis [(1a) mild, zone 3, perisinusoidal; (1b) moderate, zone 3, perisinusoidal; and (1c) portal/periportal], 2 = perisinusoidal and portal/periportal fibrosis, 3 = bridging fibrosis, and 4 = cirrhosis. According to the NASH Clinical Research Network, we calculated the NAFLD Activity Score (NAS).20 NAS is defined as the sum of the scores for steatosis (0–3), lobular inflammation (0–3), and ballooning (0–2); thus, ranging from 0 to 8. The activity score does not include liver fibrosis.
NAFL was defined by a NAS score ≤3 and NASH by a NAS score ≥4.
Enzyme-linked immunosorbent assay (ELISA)
Venous blood samples were collected in EDTA-buffered tubes after an overnight fasting. Blood samples were centrifuged at 2000 × g for 10 min using a refrigerated centrifuge and plasma aliquots were stored at −80 °C for detection.
Ang-2 plasma levels were quantified using Quantikine ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Plasma levels of intermediate filament protein fragments of CK18 were measured using the M30-Apoptosense® ELISA assay kits (PEVIVA, Sweden) according to the protocols described by the manufacturer.
The quantity of both biomarkers was calculated through plotting the standard curve in order to convert optical densities to protein concentration.
Statistical analysis
Data are given as median ± interquartile range (IQR) in the main text and tables, mean ± standard deviation (SD) in figure, and/or number (proportion) of affected patients. The Kolmogorov–Smirnov goodness-of-fit test was applied for determining whether sample data likely derived from a normally distributed population. Data had skewed distribution except anthropometric data. The nonparametric Spearman correlation coefficient, one-way analysis of variance, Bonferroni post hoc test for multiple comparisons, and regression analysis were carried out by standard techniques. Univariate analysis was performed on variables between patients with and without the study end points (NASH and fibrosis). Diagnostic accuracy of Ang-2 and CK18 were assessed by the area under the receiver operating characteristic (ROC) curve (AUC).
In the context of the present study, diagnostic accuracy refers to the ability of Ang-2 and CK18 to discriminate the presence of NASH or fibrosis. The Youden’s index was used to identify values of biomarkers with the highest accuracy that maximizes the sum of the sensitivity and specificity and the AUC value was used as the index reflecting the overall accuracy of the diagnostic test derived from the ROC analysis.
The level of significance was set at α = 0.05. SPSS 20.0 for Windows (IBM SPSS, Chicago, IL) was used for statistical analyses.
Results
Plasma levels of Ang-2 increase in pediatric patients with NAFLD
We firstly assessed the plasma levels of Ang-2 and CK18 in 28 control children without liver diseases and 76 children with biopsy-proven NAFLD. Anthropometrics, clinical, and biochemical characteristics of controls and NAFLD children are reported in Supplementary Tables 1 and 2, respectively.
We found that the mean plasma levels of both Ang-2 and CK18 in children with NAFLD were higher than in age-matched controls (Fig. 1a).
Plasma levels of Ang-2 increase in pediatric patients with NASH
Twenty-three patients presented with overweight (30%) and 54 with obesity (70%). Prevalence of co-morbidities were as follows: IFG 3.9% (3/76); IGT 13.2% (10/76) including one case with combined IFG and IGT low HDL cholesterol 30.3% (23/76); high triglycerides 17.1% (13/76); high SBP 31% (24/76); high DBP 9% (7/76). Seventeen patients (22.4%) had no metabolic abnormalities associated with obesity; 37 (48.7%) one abnormality, 19 (25%) two abnormalities, and 3 (3.9%) three abnormalities among hypertension, high triglycerides, low HDL cholesterol, IFG and/or IGT. Circulating levels of both Ang-2 and CK18 increased significantly (p = 0.04 for both) among groups with number of metabolic abnormalities increasing from none to three abnormalities.
As to histological features, steatosis was found in all biopsy specimens. Thirty-five patients (46.1%) were diagnosed with NAFL and 41 (53.9%) with NASH. Forty-seven patients (61.8%) presented with liver fibrosis of any degree and 29 had no fibrosis (38.2%).
Table 1 shows anthropometrics, clinical, and biochemical characteristics of the 76 patients (50 males, 65.8%) stratified for disease activity (NAFL, NASH, and fibrosis).
Moreover, as shown in Table 1, both Ang-2 and CK18 circulating levels increased significantly in children with NASH as compared to children with NAFL (p < 0.0001). CK18 increased significantly only in patients with fibrosis (p = 0.002).
Finally, circulating levels of Ang-2 were significantly correlated with GGT (ro = 0.314; p = 0.006) and CK18 levels (ro = 0.467; p < 0.0001).
Data on the levels of Ang-2 and CK18 in respect of the liver histologic features are reported in Table 2. Levels of Ang-2 increased significantly with worsening of steatosis, and lobular and portal inflammation. Similarly, the levels of CK18 increased significantly across the grades of steatosis, portal and lobular inflammation, NAS and fibrosis.
Diagnostic accuracy of Ang-2 and CK18 circulating levels
The overall accuracy of Ang-2 and CK18 circulating levels to diagnose NASH and fibrosis among NAFLD patients was estimated by the analysis of the AUROC. AUROC values for the levels of Ang-2 were 0.911 (95% CI 0.844–0.979; p < 0.0001) when the endpoint was the NASH (Fig. 2a), while they were 0.475 (95% CI 0.339–0.64; p = 0.720) for detecting fibrosis (Fig. 2b). AUROC values for the levels of CK18 were 0.827 (95% CI 0.735–0.919; p < 0.0001) in detecting NASH (Fig. 2c), and 0.724 (95% CI 0.611–0.837; p = 0.001) for fibrosis (Fig. 2d).
As shown in Table 3 circulating levels of Ang-2 of 135.45 ng/mL and values of CK18 of 352 IU/L were the values that predicted NASH with the best accuracy.
Discussion
This study demonstrated that Ang-2 plasma levels were increased in pediatric patients with NAFLD concurrently with the disease activity. In particular, Ang-2 levels were elevated in patients with NASH and with high grades of steatosis and portal/lobular inflammation. The ability of Ang-2 as a biomarker of disease activity was compared with that of CK18, a marker of fibrosis in children with NAFLD. Here, we found that Ang-2 levels were more efficient than CK18 levels in describing NASH, and the combining of these two biomarkers reached a positive predictive value of 100% for NASH.
Ang-2 belongs to the angiopoietin/Tie signaling system.21 Indeed. in the last decade Ang-2 has emerged, together with Ang-1, as a potent regulator of normal vascular developing, remodeling, and maturation acting as agonist and antagonist, respectively, of their common receptor, Tie2.21 Increased serum and plasma levels of Ang-2 have been documented in inflammatory diseases such as inflammatory bowel disease, and in several chronic liver diseases, including primary biliary cirrhosis and viral hepatitis B and C.22,23 Moreover, circulating levels of Ang-2 have been positively associated with inflammatory biomarkers, such as high-sensitive C-reactive protein and white blood cell count, supporting the potential use of Ang-2 as a biomarker and a putative therapeutic target.24,25 Circulating Ang-2 is also a highly sensitive and specific marker for early cardiovascular changes and cardiovascular risk in children with chronic kidney disease on dialysis.26 Finally, high blood Ang-2 levels and Ang-2/Ang-1 ratios have been associated with the presence, severity, and outcome during sepsis in newborns, children, and adults.27,28,29
Several studies reported the formation of new vessels in the liver of patients with NASH.30,31 There is an emerging body of evidence suggesting that liver sinusoidal endothelial cells (LSECs) play a critical role in inflammatory phenotype during NASH.8,32 LSECs, crucial and dynamic regulators of liver regeneration and fibrosis progression in various clinical settings, are the main source of Ang-2 in the liver.33 Indeed, circulating Ang-2 has been suggested as a non-invasive predictor of different liver fibrosis stages in patients with chronic hepatitis C.34,35 Lefere et al.,9 analyzing a sample of 104 obese patients undergoing bariatric surgery, observed that Ang-2 serum levels increased in adult patients with NASH compared with patients with simple steatosis, and found that the Ang-2 levels associated with steatosis, lobular inflammation, and ballooning, but not with fibrosis. Accordingly, we found that Ang-2 plasma levels were descriptive of NASH in our pediatric sample, where the levels of this circulating factor increased only concomitantly with the severity of lobular and portal inflammation. These findings highlight that circulating Ang-2 is a good predictor for NASH but not for fibrosis.
Moreover, we compared the trend of Ang-2 levels in children with NAFLD with the levels of CK18. CK18 fragments have been extensively studied as potential biomarkers for NASH in pediatric and adult setting.11,36 Similarly to data showed by a recent meta-analysis,37 in the present study we found that the M30 antigen, which detects caspase-cleaved CK18, had a low diagnostic accuracy for NASH. However, as previously reported by Mandelia et al.,38 in our study sample CK18 (M30) acts as a promising non-invasive biomarker for fibrosis in pediatric NAFLD. Here, we also tested the ability of the original combination of Ang-2 and CK18 circulating levels in predicting pediatric NASH. Noteworthy, our results demonstrated that the new model that combines Ang-2 and CK18 predicted NASH with a sensitivity of 71.4%, specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 80.4%.
Limitations of this study include: the fact that the study has been conducted on already available stored samples, thus this may have a little bit influence on the cut-off values; there is no correlation with other non-invasive diagnostic tools of fibrosis, such as transient elastography or hyaluronic acid; furthermore, our exploratory study was conducted in a small sample size, even if it was well-phenotyped. Our findings need validation in a general population of healthy controls and patients with obesity and various degrees of liver derangement. In future research, it would be also useful to test the performance of both biomarkers with respect to liver stiffness measured by elastography. Indeed, while we expect that combination of these two tests predicts with high accuracy NASH in an external large sample of children with NAFLD, we cannot rule out accuracy of both testing in a general population of patients with obesity.
In conclusion, our findings suggest a potential role of Ang-2 as a biomarker of NASH also in a pediatric setting, thus adding a novel valuable tool for non-invasive stratification of NAFLD patients.
References
Younossi, Z. M. Non-alcoholic fatty liver disease—a global public health perspective. J. Hepatol. 70, 531–544 (2019).
Nobili, V. et al. NAFLD in children: new genes, new diagnostic modalities and new drugs. Nat. Rev. Gastroenterol. Hepatol. 16, 517–530 (2019).
Estes, C., Razavi, H., Loomba, R., Younossi, Z. & Sanyal, A. J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 67, 123–133 (2018).
Ballestri, S. et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 31, 936–944 (2016).
Marra, F. & Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 68, 280–295 (2018).
Povero, D. & Feldstein, A. E. Novel molecular mechanisms in the development of non-alcoholic steatohepatitis. Diabetes Metab. J. 40, 1–11 (2016).
Gehrke, N. & Schattenberg, J. M. Metabolic inflammation—a role for hepatic inflammatory pathways as driver of comorbidities in non-alcoholic fatty liver disease (NAFLD)? Gastroenterology 158, 1929–1947 (2020).
Hammoutene, A. & Rautou, P. E. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J. Hepatol. 70, 1278–1291 (2019).
Lefere, S. et al. Angiopoietin-2 promotes pathological angiogenesis and is a novel therapeutic target in murine non-alcoholic fatty liver disease. Hepatology 69, 1087–1104 (2019).
Pauta, M. et al. Overexpression of angiopoietin-2 in rats and patients with liver fibrosis. Therapeutic consequences of its inhibition. Liver. Int. 35, 1383–1392 (2015).
Mosca, A., Panera, N., Crudele, A. & Alisi, A. Noninvasive diagnostic tools for pediatric NAFLD: where are we now? Expert. Rev. Gastroenterol. Hepatol. 14, 1035–1046 (2020).
Vajro, P. et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J. Pediatr. Gastroenterol. Nutr. 54, 700–713 (2012).
Farpour-Lambert, N. J. et al. Childhood obesity is a chronic disease demanding specific health care–a position statement from the Childhood Obesity Task Force (COTF) of the European Association for the Study of Obesity (EASO). Obes. Facts 8, 342–349 (2015).
Cacciari, E. et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Invest. 29, 581–593 (2006).
Matsuda, M. & DeFronzo, R. A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999).
Štěpánek, L. et al. Associations between homeostasis model assessment (HOMA) and routinely examined parameters in individuals with metabolic syndrome. Physiol. Res. 68, 921–930 (2019).
Flynn, J. T. et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140, e20171904 (2017).
Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 128 (Suppl. 5), S213–S256 (2011).
Brunt, E. M. et al. Portal chronic inflammation in non-alcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-clinicopathologic correlations from the non-alcoholic steatohepatitis clinical research network. Hepatology 49, 809–820 (2009).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Akwii, R. G., Sajib, M. S., Zahra, F. T. & Mikelis, C. M. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells 8, 471 (2019).
Voigtländer, T. et al. Angiopoietin-2 and biliary diseases: elevated serum, but not bile levels are associated with cholangiocarcinoma. PLoS ONE 9, e97046 (2014).
Hernández-Bartolomé, Á. et al. Angiopoietin-2/angiopoietin-1 as non-invasive biomarker of cirrhosis in chronic hepatitis C. World J. Gastroenterol. 22, 9744–9751 (2016).
Schuldt, E. A. et al. Circulating angiopoietin-2 and its soluble receptor Tie-2 concentrations are related to inflammatory markers in the general population. Cytokine 105, 1–7 (2018).
Huang, H., Bhat, A., Woodnutt, G. & Lappe, R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat. Rev. Cancer 10, 575–585 (2010).
Abdel-Salam, M. et al. Evaluation of angiopoietin-2 serum level as a marker of cardiovascular risk in children with chronic kidney disease. Open J. Nephrol. 5, 105–116 (2015).
Wang, K. et al. Angiopoietin-1, angiopoietin-2 and bicarbonate as diagnostic biomarkers in children with severe sepsis. PLoS ONE 9, e108461 (2014).
Ricciuto, D. R. et al. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit. Care. Med. 39, 702–710 (2011).
Zonneveld, R. et al. Low serum Angiopoietin-1, high serum Angiopoietin-2, and high Ang-2/Ang-1 protein ratio are associated with early onset sepsis in Surinamese newborns. Shock 48, 638–643 (2017).
Coulon, S. et al. Evaluation of inflammatory and angiogenic factors in patients with non-alcoholic fatty liver disease. Cytokine 59, 442–449 (2012).
Coulon, S. et al. Role of vascular endothelial growth factor in the pathophysiology of nonalcoholic steatohepatitis in two rodent models. Hepatology 57, 1793–1805 (2013).
Miyao, M. et al. Pivotal role of liver sinusoidal endothelial cells in NAFLD/NASH progression. Lab. Invest. 95, 1130–1144 (2015).
Wang, R., Huebert, R. C. & Shah, V. H. Sinusoidal endothelial cells coordinate liver regeneration and angiogenesis via angiopoietin-2: an ode to prometheus. Gastroenterology 147, 533–534 (2014).
Hernández-Bartolomé, A. et al. Angiopoietin-2 serum levels improve noninvasive fibrosis staging in chronic hepatitis C: a fibrogenic-angiogenic link. PLoS ONE 8, e66143 (2013).
Makhlouf, M. M. et al. Serum angiopoietin-2 as a noninvasive diagnostic marker of stages of liver fibrosis in chronic hepatitis C patients. Egypt. J. Intern. Med. 28, 140–148 (2016).
Feldstein, A. E. et al. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology 50, 1072–1078 (2009).
Lee, J. et al. Accuracy of cytokeratin 18 (M30 and M65) in detecting non-alcoholic steatohepatitis and fibrosis: a systematic review and meta-analysis. PLoS ONE 15, e0238717 (2020).
Mandelia, C. et al. Plasma cytokeratin-18 level as a novel biomarker for liver fibrosis in children with nonalcoholic fatty liver disease. J. Pediatr. Gastroenterol. Nutr. 63, 181–187 (2016).
Acknowledgements
The authors would like to thank our nursing staff members (Giulia Pace, Anna Maria Conti, and Loriana Giammaria) for their precious assistance in caring patients and in recruiting them for this project.
Funding
This work was supported by Italian Ministry of Health (Fondi-di-Ricerca Corrente 2020) to A.A.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.M., N.P. and A.A.; methodology: A.C., D.C., M.B. and R.D.V.; formal analysis: M.M., N.P., A.C. and M.R.B.; data curation: M.M., N.P., M.R.B., M.B., R.D.V., G.M. and A.A.; writing—original draft preparation: N.P., D.C. and G.M.; writing—review and editing: M.M. and A.A.; supervision and project administration: A.A.; funding acquisition: A.A. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
The consent was obtained for biobanking of anonymized samples and use in future investigations relating to the same topic.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Manco, M., Panera, N., Crudele, A. et al. Angiopoietin-2 levels correlates with disease activity in children with nonalcoholic fatty liver disease. Pediatr Res 91, 1781–1786 (2022). https://doi.org/10.1038/s41390-021-01666-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01666-5