Abstract
Background
Stem cell therapy has been proven to rescue intestinal injury and stimulate intestinal regeneration in necrotizing enterocolitis (NEC). Specifically, stem cells derived from amniotic fluid (AFSCs) and mesenchymal stem cells (MSCs) derived from bone marrow have shown promising results in the treatment of experimental NEC. This study aims to examine the effects of AFSCs and MSCs on the prevention of intestinal injury during experimental NEC.
Methods
Supernatants from AFSC and MSC cultures were collected to perform proteomic analysis. Prior to NEC induction, mice received intraperitoneal injections of phosphate-buffered saline (PBS), 2 × 106 AFSCs, or 2 × 106 MSCs.
Results
We found that AFSCs grew faster than MSCs. Proteomic analysis indicated that AFSCs are primarily involved in cell development and growth, while MSCs are involved in immune regulation. Administering AFSCs before NEC induction decreased NEC severity and mucosal inflammation. Intestinal proliferation and endogenous stem cell activation were increased after AFSC administration. However, administering MSCs before NEC induction had no beneficial effects.
Conclusions
This study demonstrated that AFSCs and MSCs have different protein release profiles. AFSCs can potentially be used as a preventative strategy for neonates at risk of NEC, while MSCs cannot be used.
Impact
-
AFSCs and MSCs have distinct protein secretory profiles, and AFSCs are primarily involved in cell development and growth, while MSCs are involved in immune regulation.
-
AFSCs are unique in transiently enhancing healthy intestinal epithelial cell growth, which offers protection against the development of experimental NEC.
-
The prevention of NEC via the administration of AFSCs should be evaluated in infants at great risk of developing NEC or in infants with early signs of NEC.
Similar content being viewed by others
Introduction
Necrotizing enterocolitis (NEC) is one of the most devastating diseases in newborns, primarily affecting preterm and low birth weight neonates.1,2 There are currently no specific medical treatments for infants with NEC, and surgical resection of affected segments often leads to short bowel syndrome and/or intestinal failure.3 Despite extensive research on the prevention of NEC and significant improvements in neonatal care over the last three decades, NEC incidence and mortality remain high,4 indicating an urgent need for innovative prevention strategies.
Experimental studies using animal models of NEC indicate that various types of stem cells protect the intestine from the severe damage caused by NEC.5,6,7,8,9 Amniotic fluid-derived stem cells (AFSCs) isolated from humans and rodents grow rapidly and express both embryonic and adult stem cell markers.10 AFSCs are similar to embryonic stem cells (ESCs), explaining their capacity for pluripotency and rapid growth.11 In an experimental rodent NEC model, we reported that AFSC administration improved survival, clinical status, intestinal structure, and intestinal function by decreasing apoptosis and inflammation and increasing proliferation and migration. These changes are thought to occur through a paracrine mechanism.9,12
Mesenchymal stem cells (MSCs) are adult multipotent cells that can differentiate into a variety of different cell types and have been studied extensively in both animal models and humans. MSCs can be readily derived from donors, most commonly from bone marrow, and can be cultured in vitro. It has been demonstrated that MSCs derived from rats and humans can effectively reduce the incidence and severity of experimental NEC in mouse and rat models.5,6
In experimental studies,5,6,7,8,9 stem cells were typically administered during NEC induction, making it difficult to distinguish whether stem cell administration is effective in preventing or treating the disease. This is further complicated by the requirement of 2–3 days to develop villi detachment and mucosal inflammation in experimental NEC. In contrast, impaired intestinal perfusion develops rapidly and can be detected as early as within the first day of NEC induction.13 To our knowledge, the preventative potential of stem cell administration in experimental NEC has not been properly investigated. Therefore, to provide a proof of concept, our experimental study focused on assessing whether AFSCs and/or MSCs play an important role in the prevention of NEC. The prevention of NEC by the administration of stem cells or their products should be evaluated, as it may be of benefit to infants with “suspected” NEC, such as Bell’s stage I.14,15
We hypothesize that AFSCs and MSCs have different protective effects against NEC-induced intestinal epithelium injury. In this study, we demonstrated that the supernatant from AFSCs contained proteins involved in cellular, developmental, and metabolic processes, whereas the proteins released by MSCs were involved in immune system processes. Our data indicate that AFSCs, but not MSCs, administered before the onset of NEC can prevent the development of intestinal epithelial injury.
Materials and methods
Stem cell isolation and culture
Stem cell isolation and characterization were based on previously published protocols in which stem cells were used in experimental NEC.9,16 Briefly, for AFSCs amniotic fluid was harvested from pregnant rats on day 14.5 of gestation via aspiration of amniotic sacs using a 25-gauge needle, as previously described.10,17 C-kit (CD-117)-positive selected AFSCs were subsequently grown in α-minimum essential medium (α-MEM) (Gibco) supplemented with 20% Chang medium C (Irvine Scientific), 15% fetal bovine serum (FBS, Thermo Fisher), and 1% penicillin/streptomycin (PSA, Sigma-Aldrich). For MSCs femur and tibia bones of adult rats were cleared of the surrounding tissue, and the ends were clipped with bone-cutting forceps. The marrow was flushed and cultured with 2 ml of culture medium consisting of α-MEM, 10% FBS, and 1% PSA.
Proteomics
To mitigate interference by FBS, stem cells were cultured in a serum-free medium for 24 h, and the culture medium supernatant was harvested and concentrated using an Amicon Ultra 4 centrifugal filter tube (Millipore). Proteomic analyses were performed by the Center for Advanced Proteomics Analyses (Montreal, Canada) using standard protocols. Mass spectrometry was acquired with a resolution of 70,000 using a lock mass (m/z: 445.120025), followed by up to 10 MS/MS data-dependent scans on the most intense ions using high-energy dissociation (HCD). AGC target values for MS and MS/MS scans were set to 1e6 (max fill time 500 ms) and 1e6 (max fill time 120 ms), respectively. The precursor isolation window was set to m/z: 2 with an HCD-normalized collision energy of 25. The dynamic exclusion window was set to 30 s. MS data were analyzed using Scaffold 4 software version 1.3.0.3; http://www.UniProt.org/). Serum-free medium served as the baseline, and MSC- or AFSC-enriched conditioned medium was compared with baseline serum-free medium.
NEC induction
All animal experiments were approved by the Animal Care Committee at The Hospital for Sick Children (protocol no. 44032), and all methods were performed according to the guidelines and regulations. NEC was induced as described previously9,18 in 5-day-old neonatal C57BL/6 mice using gavage feeding of formula, hypoxia, and oral lipopolysaccharide (4 mg/kg) for 4 days (postnatal days 5–9). Breastfed mice served as controls (n = 10). Several litters were used, and pups from each litter were randomly assigned to each of the four experimental groups (control, NEC, AFSC + NEC, and MSC + NEC) to eliminate potential differences between litters and litter effects. On postnatal days 3 and 4 prior to NEC induction, mice received an intraperitoneal injection of phosphate-buffered saline (PBS; n = 10), 2 × 106 AFSCs (n = 10), or 2 × 106 MSCs (n = 10). The dose of stem cells administered over 2 days was chosen based on previous studies reporting the successful therapeutic effect of AFSCs.9,12 This allows the comparison of different studies with respect to potential prevention and treatment. On postnatal day 9, pups were sacrificed, and the distal ileum was harvested.
An additional experiment was performed to rule out hyperproliferation induced by AFSC administration. Mouse pups were randomly assigned to four experimental groups (six animals per group): (1) controls sacrificed for evaluation at P4; (2) controls receiving AFSCs at P3 and P4 and evaluated at P4; (3) controls evaluated at P9; and (4) controls receiving AFSCs at P3 and P4 and evaluated at P9. Animals from groups 2 and 4 received an intraperitoneal injection of PBS and 2 × 106 AFSCs. All animals returned to the dam for breastfeeding after AFSC administration.
Histopathology
After sacrificing the mouse pups, 1-cm-long distal ileal samples were collected and fixed in 4% paraformaldehyde. The intestinal tissues were embedded in paraffin, cross-sectioned (5 µm), and stained with hematoxylin and eosin. Histological sections were assessed by three blinded investigators following an established NEC histopathological scoring system, and mice with NEC grade ≥2 were considered NEC-positive.19,20
Immunostaining
Cells were fixed using 4% paraformaldehyde and subsequently permeabilized with 0.1% Triton X-100. After blocking, the cells were incubated overnight at 4 °C with CD34, CD133, Oct4, and Sox2 primary antibodies (Invitrogen, Carlsbad, CA). Cells were then incubated with an Alexa Fluor-conjugated secondary antibody (Invitrogen) and 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories) at room temperature for 2 h.
Sections of terminal ileum were immunostained with 1:500 dilutions of primary antibodies for Ki67, followed by incubation with 1:1000 diluted secondary antibodies and DAPI (Vector Laboratories, Burlington, ON) for visualization of cell nuclei. Staining was analyzed using a Nikon TE-2000 digital microscope equipped with a Hamamatsu C4742-80-12AG camera. As previously described,12,21 Ki67 staining is an intranuclear stain providing clear demarcation of stained nuclei. Therefore, to obtain an accurate measurement, manual counting was conducted by three blinded investigators using 5–15 images for each animal and measuring 15–25 crypts/villi in each image to provide an appropriate representation of the number of antibody-labeled cells per image.
Gene quantification
RNA was isolated from the distal ileum with TRIzol (Invitrogen). Total RNA (1 µg) was reverse transcribed using qScript cDNA Supermix (Quanta Biosciences, Gaithersburg). SYBR Green-based real-time quantitative reverse transcription PCR was performed using a CF384 C1000 Thermal Cycler (Bio-Rad) and Evagreen Supermix (Bio-Rad) using previously described primers and conditions for IL-6 and Lgr5.22 Data were analyzed using CFX Manager 3.1 (Bio-Rad). The results are from three independent experiments performed in triplicate. Expression levels were calculated by the ∆∆Ct method and normalized to the reference housekeeping genes GAPDH and RPL0.
Statistics
The results are presented as the means ± SD, as data were normally distributed (Kolmogorov–Smirnov test). Groups were compared using Student’s t test or one-way analysis of variance with post hoc correction as appropriate. P < 0.05 was considered statistically significant.
Results
AFSCs and MSCs have distinct secretory profiles
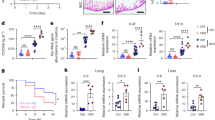
AFSCs and bone marrow-derived MSCs were isolated from rats. Both AFSCs and MSCs expressed pluripotent stem cell markers such as Oct4 and Sox2. Similar to a previous study,10 AFSCs were negative for CD34 and CD133, which are markers of hematopoietic stem cells. MSCs were also CD34-negative but CD133-positive. Upon culturing, AFSCs grew faster than bone marrow-derived MSCs (Supplementary Fig. 1 and Fig. 1a). AFSC and MSC serum-free culture supernatants were collected, and mass spectrometry was performed to compare the secreted proteomes of these two stem cell types to the baseline serum-free medium. Gene ontology (GO) comparison of proteins present in the supernatants of AFSCs and MSCs revealed that AFSC-secreted proteins were primarily involved in biological adhesion, cellular processes, development, growth, metabolism, and reproduction, while MSC-secreted proteins were primarily involved in immune system-related processes (Fig. 1b). AFSC-secreted proteins were highly involved in the regulation of cell growth and cell size (Fig. 1c and Table 1). These differences in growth rates and protein secretory profiles suggest that AFSCs and MSCs can have different effects on stem cell-mediated preventative strategies.
a Growth rates of AFSCs and MSCs over 13 days of culture. b Gene ontology of biological processes comparing AFSCs and MSCs. c AFSC-secreted proteins are involved in a variety of developmental processes. Samples for proteomic analysis were collected from three independently repeated experiments. Data are presented as means ± SD. ***p < 0.001, using Student’s t test.
AFSCs, not MSCs, prevent intestinal injury during experimental NEC
We next injected AFSCs and MSCs into mouse pups prior to inducing experimental NEC to determine whether stem cells (AFSCs or MSCs) could prevent the development of the disease. NEC-like intestinal injury was eliminated by the preventive administration of AFSCs, whereas no changes were observed after the administration of MSCs (Fig. 2a, c). Seventy percent of mouse pups developed grade 2+ NEC-like injury in both the PBS + NEC group (7/10) and the MSC + NEC group (6/10) compared to none of the pups in the AFSC + NEC group (0/10; p < 0.001; Fig. 2c). The histological score for pups pretreated with AFSCs was significantly lower than that for pups pretreated with MSCs, with the AFSC pretreatment group having a histological score similar to that of the control group (Fig. 2c). Similarly, the messenger RNA expression of the inflammatory cytokine interleukin-6 was significantly reduced in the AFSC pretreatment group compared to pups pretreated with PBS or MSCs (Fig. 2d). The intestinal epithelial proliferation marker Ki67 (Fig. 2b, e) and intestinal stem cell marker Lgr5 (Fig. 2f) were both elevated in the AFSC pretreatment group but not in the MSC pretreatment group. These data suggest that administration of AFSCs, and not MSCs, prior to the onset of experimental NEC prevents NEC-induced intestinal injury by prompting intestinal growth.
a Representative H&E-stained histomicrographs and b Ki67-stained micrographs of the control, PBS + NEC, AFSC + NEC, and MSC + NEC groups. c Histological score for each group. d Relative gene expression of the inflammation marker IL-6. e Quantification of Ki67-positive cells per crypt for all experimental groups. f Relative gene expression for ISC marker Lgr5. Data are presented as means ± SD. There were ten mouse pups in each group. **p < 0.01; ***p < 0.001, using one-way ANOVA with post hoc tests.
Furthermore, to evaluate whether the observed increase in proliferation after AFSC administration was transient, AFSCs were administered to breastfed control pups, and intestinal proliferation was assessed at postnatal days 4 and 9. Compared to controls not receiving AFSCs, there was a significant increase in proliferation at postnatal day 4 but no proliferative changes at postnatal day 9 (Supplementary Fig. 2).
Discussion
In this study, we demonstrated that AFSCs and MSCs have distinct characteristics and effects on the intestinal epithelium during experimental NEC. AFSCs and MSCs differ in terms of growth rates and secretory protein profiles. The analysis of proteins released from these stem cells indicated that AFSCs are involved in cell development and growth, while MSCs play a role in immune regulation. Mouse pups pretreated with AFSCs, but not MSCs, showed increased intestinal growth and decreased NEC-induced injury (Fig. 3).
AFSCs have exceptionally high expression levels of ESC markers, which may explain their greater capacity for proliferation, multipotency, and immunomodulatory activities than MSCs.10,23,24 Fortunately, unlike ESCs, AFSCs remain stable and show no signs of transformation in culture.9,10 In particular, AFSCs were not observed to form tumors when injected into immune-deficient mice.10 Due to their fetal but nonembryonic origin, embryonic-like AFSCs circumvent many concerns that arise from the use of ESCs. This unique characteristic of AFSCs warrants further investigation into the use of AFSCs in disease treatment and prevention. Extensive research has demonstrated the value of AFSC treatment in various intestinal diseases.9,12,25 However, this study reported that AFSCs have an additional advantage of being active under healthy conditions where there is no notable intestinal damage. AFSCs appear to stimulate the proliferation of epithelial cells and increase the number of functional Lgr5+ stem cells, leading to the prevention of intestinal injury and inflammation. Interestingly, this increase in proliferation appears to be a transient phenomenon, and AFSC administration does not appear to cause continuing hyperproliferation. Our data indicate that increased epithelium proliferation was observed immediately after the injection of AFSCs at postnatal day 4 but was not maintained at postnatal day 9 in the breastfed control mice (Supplementary Fig. 2). These findings support the concept that AFSC administration stimulates an increase in intestinal proliferation in a short time window and that proliferation returns to normal levels afterward.
Recent studies have focused on identifying secretory factors derived from AFSCs that might serve as a viable option for the treatment of various diseases. In this context, we performed a proteomic analysis to compare the protein profiles of AFSCs and MSCs. Functional analysis of the AFSC-secreted proteins (Table 1) suggested that this stem cell type is involved in the regulation of several pathways for cell growth and metabolism. This is consistent with our finding that AFSCs can stimulate intestinal proliferation and intestinal stem cell preservation under both healthy and injured conditions. Among the AFSC-secreted proteins were the serine/threonine-protein phosphatase PP1-γ catalytic subunit and the insulin growth factor-binding protein (IGFBP) family. The PP1-γ subunit is involved in a wide range of cellular processes, especially meiosis and cell division, protein synthesis, glycogen metabolism, cytoskeletal reorganization, and regulation of membrane receptors and channels.26 The IGFBP superfamily has been shown to modulate survival, migration, and proliferation in many cells.27,28 IGFBP3, the most abundant protein of the IGFBP family, is a multifunctional protein released from cells that regulates cell proliferation and apoptosis in an insulin-like growth factor-dependent and insulin-like growth factor-independent manner.29,30 IGFBP3 has been implicated in promoting cell growth and other cell functions depending on specific conditions.31,32 Notably, IGFBP3 has been reported to interact with epidermal growth factor receptor,33 which is involved in the treatment of NEC.34 Other members of the IGFBP family, such as IGFBP-7, have been shown to be responsible for the increased migration and proliferation of epidermal keratinocytes in an in vitro scratch assay.35 This is consistent with our finding that AFSCs promote enhanced cell migration and result in a significantly smaller wound gap under normal conditions. Due to the limited sensitivity of the proteomic analysis, other factors that remain undetected may also play an important role in cell growth. Indeed, AFSCs have been shown to secrete significantly more growth factors than MSCs, including fibroblast growth factor, vascular endothelial growth factor, hepatocyte growth factor, and the IGFBP superfamily.36 In another study, a higher level of Wnt expression was observed in AFSCs than in MSCs, suggesting that AFSCs have a more prominent role in regulating intestinal stem cell proliferation.9
In this study, we did not trace where the injected stem cells traveled. However, a prior study that examined prophylactic administration of stem cells in a rodent septic model37 showed that AFSCs transiently accumulate in the liver, mesentery, and peritoneum after injection, followed by the release of paracrine factors and induction of M1 to M2 macrophage polarization in a cell–cell contact-independent manner. Moreover, the maturation of M2 macrophages has been reported to play an important role in stimulating intestinal stem cells to maintain intestinal hemostasis.38 These findings suggest that the beneficial effects of AFSCs are less likely to be related to their localization but rather to the paracrine factors derived from AFSCs. This is in line with our previous findings in experimental NEC treatment using AFSCs.9,12 Further investigations to identify the paracrine factors released by AFSCs are required to be beneficial in preventing and/or treating patients with NEC.
In a side-by-side comparison of the administration of different stem cell types in an experimental study using a rat model, AFSCs and MSCs were equivalently effective in reducing intestinal injury in NEC.16,39 In our current mouse model, AFSCs had an additional advantage over MSCs in being active under healthy conditions where there was no notable intestinal damage. This difference may be attributed to the rat pups in the studies being delivered 0.5 days prematurely by C-section, making it difficult to know the baseline intestinal injury level and inflammation when exogenous stem cells were administered. In our current mouse study, stem cell administration is known to act prophylactically because the administration of stem cells occurs prior to the induction of any intestinal injury. In addition, the native intestinal stem cell population may differ in premature rats and postnatal day 5 mice. This adds another possible explanation for why the different exogenous stem cell types may be similarly effective in premature rats, as they may replenish a diminished intestinal stem cell population, whereas in postnatal day 5 normal mice, there may be no such deficiency.
There are some limitations in our study. First, we noted that mouse AFSCs and MSCs would provide an ideal comparison platform. However, mouse MSCs are difficult to expand and detach in cell culture.40,41 Thus, in our study, we used stem cells derived from rats. This strategy raises a potential concern about the use of the mouse model of NEC over the rat model of NEC. Induction of NEC in rats typically starts at postnatal day 0,8,39 which allows no time window to perform preventive treatment prior to NEC induction. Second, our study only provides a proof of concept that identified the capability of AFSCs to act in a beneficial manner in NEC prevention. Subsequent studies are required to explore the mechanism of action, the magnitude of the effect, and the window of opportunity for preventative AFSC treatment. Paracrine factors will also need to be further evaluated. In addition, the route of administration, target population, and how these findings can safely be incorporated into clinical trials of human patients need to be examined.
In summary, AFSCs and MSCs have distinct secretory protein profiles. AFSCs have the unique advantage of transiently enhancing healthy intestinal epithelial cell growth, which offers protection against the development of experimental NEC. Translation of these exciting novel observations into humans is of paramount importance. Prevention of NEC by the administration of AFSC and its derivatives should be evaluated in infants at great risk of developing NEC or in infants with early signs of NEC.
References
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011).
Yee, W. H. et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 129, e298–e304 (2012).
Pierro, A. The surgical management of necrotising enterocolitis. Early Hum. Dev. 81, 79–85 (2005).
Thyoka, M. et al. Advanced necrotizing enterocolitis part 1: mortality. Eur. J. Pediatr. Surg. 22, 8–12 (2012).
Tayman, C. et al. Mesenchymal stem cell therapy in necrotizing enterocolitis: a rat study. Pediatr. Res. 70, 489–494 (2011).
Yang, J. et al. Heparin-binding epidermal growth factor-like growth factor and mesenchymal stem cells act synergistically to prevent experimental necrotizing enterocolitis. J. Am. Coll. Surg. 215, 534–545 (2012).
Wei, J., Zhou, Y. & Besner, G. E. Heparin-binding EGF-like growth factor and enteric neural stem cell transplantation in the prevention of experimental necrotizing enterocolitis in mice. Pediatr. Res. 78, 29–37 (2015).
Zani, A. et al. Amniotic fluid stem cells prevent development of ascites in a neonatal rat model of necrotizing enterocolitis. Eur. J. Pediatr. Surg. 24, 57–60 (2014).
Zani, A. et al. Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut 63, 300–309 (2014).
De Coppi, P. et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 25, 100–106 (2007).
Arnhold, S. et al. Amniotic-fluid stem cells: growth dynamics and differentiation potential after a CD-117-based selection procedure. Stem Cells Int. 2011, 715341 (2011).
Li, B. et al. Activation of Wnt signaling by amniotic fluid stem cell-derived extracellular vesicles attenuates intestinal injury in experimental necrotizing enterocolitis. Cell Death Dis. 11, 750 (2020).
Koike, Y. et al. Remote ischemic conditioning counteracts the intestinal damage of necrotizing enterocolitis by improving intestinal microcirculation. Nat. Commun. 11, 4950 (2020).
Walsh, M. C. & Kliegman, R. M. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr. Clin. N. Am. 33, 179–201 (1986).
Bell, M. J. et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 187, 1–7 (1978).
McCulloh, C. J. et al. Evaluating the efficacy of different types of stem cells in preserving gut barrier function in necrotizing enterocolitis. J. Surg. Res. 214, 278–285 (2017).
Antounians, L. et al. The regenerative potential of amniotic fluid stem cell extracellular vesicles: lessons learned by comparing different isolation techniques. Sci. Rep. 9, 1837 (2019).
Zani, A. et al. A spectrum of intestinal injury models in neonatal mice. Pediatr. Surg. Int. 32, 65–70 (2016).
Dvorak, B. et al. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am. J. Physiol. Gastrointest. Liver Physiol. 282, G156–G164 (2002).
Ran-Ressler, R. R. et al. Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS ONE 6, e29032 (2011).
Li, B. et al. Impaired Wnt/beta-catenin pathway leads to dysfunction of intestinal regeneration during necrotizing enterocolitis. Cell Death Dis. 10, 743 (2019).
Li, B. et al. Inhibition of corticotropin-releasing hormone receptor 1 and activation of receptor 2 protect against colonic injury and promote epithelium repair. Sci. Rep. 7, 46616 (2017).
Pozzobon, M., Piccoli, M., Schiavo, A. A., Atala, A. & De Coppi, P. Isolation of c-Kit+ human amniotic fluid stem cells from second trimester. Methods Mol. Biol. 1035, 191–198 (2013).
Schiavo, A. A. et al. Endothelial properties of third-trimester amniotic fluid stem cells cultured in hypoxia. Stem Cell Res. Ther. 6, 209 (2015).
Koike, Y. et al. The intestinal injury caused by ischemia-reperfusion is attenuated by amniotic fluid stem cells via the release of tumor necrosis factor-stimulated gene 6 protein. FASEB J. 34, 6824–6836 (2020).
Ceulemans, H. & Bollen, M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiological Rev. 84, 1–39 (2004).
Firth, S. M. & Baxter, R. C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 23, 824–854 (2002).
Ferry, R. J. Jr., Katz, L. E., Grimberg, A., Cohen, P. & Weinzimer, S. A. Cellular actions of insulin-like growth factor binding proteins. Horm. Metab. Res. 31, 192–202 (1999).
Grimberg, A. & Cohen, P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J. Cell. Physiol. 183, 1–9 (2000).
Jogie-Brahim, S., Feldman, D. & Oh, Y. Unraveling insulin-like growth factor binding protein-3 actions in human disease. Endocr. Rev. 30, 417–437 (2009).
Granata, R. et al. Dual effects of IGFBP-3 on endothelial cell apoptosis and survival: involvement of the sphingolipid signaling pathways. FASEB J. 18, 1456–1458 (2004).
Chang, K. H. et al. IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc. Natl Acad. Sci. USA 104, 10595–10600 (2007).
Takaoka, M. et al. Epidermal growth factor receptor regulates aberrant expression of insulin-like growth factor-binding protein 3. Cancer Res. 64, 7711–7723 (2004).
Good, M. et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 8, 1166–1179 (2015).
Nousbeck, J. et al. Insulin-like growth factor-binding protein 7 regulates keratinocyte proliferation, differentiation and apoptosis. J. Investig. Dermatol. 130, 378–387 (2010).
Skardal, A. et al. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl. Med. 1, 792–802 (2012).
Sato, Y. et al. Prophylactic therapy with human amniotic fluid stem cells improved survival in a rat model of lipopolysaccharide-induced neonatal sepsis through immunomodulation via aggregates with peritoneal macrophages. Stem Cell Res. Ther. 11, 300 (2020).
Thomas, H. Intestinal homeostasis is reliant on self-maintaining macrophages. Nat. Rev. Gastroenterol. Hepatol. 15, 656–657 (2018).
McCulloh, C. J., Olson, J. K., Zhou, Y., Wang, Y. & Besner, G. E. Stem cells and necrotizing enterocolitis: a direct comparison of the efficacy of multiple types of stem cells. J. Pediatr. Surg. 52, 999–1005 (2017).
Dvorakova, J., Hruba, A., Velebny, V. & Kubala, L. Isolation and characterization of mesenchymal stem cell population entrapped in bone marrow collection sets. Cell Biol. Int. 32, 1116–1125 (2008).
Zhu, H. et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat. Protoc. 5, 550–560 (2010).
Acknowledgements
Proteomic analyses were performed by the Center for Advanced Proteomics Analyses, a Node of the Canadian Genomic Innovation Network that is supported by the Canadian Government through Genome Canada. B.L. is the recipient of a Restracomp Fellowship, The Hospital for Sick Children, and an Early Career Award Program grant from the Thrasher Research Fund (14503). A.P. is the recipient of a Canadian Institutes of Health Research (CIHR) Foundation Grant (353857) and was also supported by the Robert M. Filler Chair of Surgery, The Hospital for Sick Children.
Author information
Authors and Affiliations
Contributions
B.L., C.L., M.C., J.S.O’C., S.R.B., H.M., M.A., N.G., K.C.J.-H., and P.M.S.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approval of the manuscript. P.M. and A.P.: conception and design, financial support, and final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Patient consent was not required for this experimental study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, B., Lee, C., Cadete, M. et al. Amniotic fluid stem cell administration can prevent epithelial injury from necrotizing enterocolitis. Pediatr Res 91, 101–106 (2022). https://doi.org/10.1038/s41390-021-01657-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01657-6
This article is cited by
-
Necrotizing enterocolitis: current understanding of the prevention and management
Pediatric Surgery International (2024)
-
Stem cell therapy as a promising strategy in necrotizing enterocolitis
Molecular Medicine (2022)