Abstract
Background
We investigated the role of inhibitory receptors (IRs) and especially lymphocyte activation gene-3 (LAG-3) in the pathogenesis of oligoarticular juvenile idiopathic arthritis (o-JIA).
Methods
Paired samples of synovial fluid (SF) and plasma and peripheral blood (PBMCs) and synovial fluid mononuclear cells (SFMCs) were collected from o-JIA patients along with their clinical data (n = 24). Plasma from healthy controls (n = 14) and paired SF and plasma samples from five non-arthritic juvenile orthopedic patients (n = 5) served as controls. Spontaneously differentiated fibroblast-like synoviocytes (FLSs) from SFMCs were co-cultured with autologous PBMCs/SFMCs and used as ex vivo disease model. Soluble levels and cellular expressions of IRs together with their functional properties in the ex vivo model were analyzed.
Results
In patients with o-JIA, soluble levels of LAG-3 and expression of LAG-3 and T cell immunoglobulin mucin03 (TIM-3) on CD3+CD4+CD45RO+ T cells were increased, especially in SF. Major histocompatibility complex (MHC) class II expression was induced on FLSs when these were co-cultured with autologous PBMCs/SFMCs, together with an increased monocyte chemoattractant protein-1 (MCP-1) production. In PBMC and FLS + PBMC co-cultures, neutralizing antibodies to IRs were added. Only anti-LAG-3 antibodies significantly increased MCP-1 secretion. The addition of agonistic LAG-3 antibody resulted in decreased effector cytokine secretion.
Conclusions
This is the first report comparing the effects of different IRs in o-JIA and suggests that LAG-3 might contribute to the pathogenesis of this disease.
Impact
-
This is the first study addressing the role of different co-IRs in o-JIA.
-
We showed that LAG-3 and TIM-3 seem more important in juvenile arthritis in contrast to adult rheumatoid arthritis, where cytotoxic T-lymphocyte-associated antigen-4 and programmed cell death-1 were reported to be more important.
-
We designed an ex vivo disease model for o-JIA, examined the effects of co-IRs in this model, and demonstrated that they might contribute to the pathogenesis of the disease.
-
LAG-3 might play a role in o-JIA pathogenesis and might be a potential therapeutic option for o-JIA patients.
Similar content being viewed by others
Introduction
Juvenile idiopathic arthritis (JIA) is a chronic inflammatory disease that can cause severe disability and joint damage.1 Using the International League of Associations for Rheumatology (ILAR) criteria, it is possible to divide patients into six subgroups according to specific features and the number of joints affected.2 JIA is one of the most common inflammatory joint diseases in children, with an incidence of 16–150/100,000.1,2,3,4 Previous studies revealed that T cells play a central role in the pathogenesis of oligoarticular and polyarticular forms of the disease. Furthermore, recent genome-wide association studies of very large JIA cohorts revealed a close link between both major histocompatibility complex (MHC) class I (HLA-A2 and HLA-B27), class II (HLA-DRB1 and HLA-DP) and, PTPN22, all associated with the adaptive immune system response.5,6,7 The involvement of continued T cell activation in JIA pathogenesis is further supported by the accumulation of activated T cells in the synovial fluid (SF) and the efficacy of T cell-targeted therapies in JIA.8,9
Recent studies have shown that the balance between T cell co-stimulatory and co-inhibitory receptors (IRs) is important in the adaptive immune system’s response. T cell IRs seem to play an important role in the development of tolerance and in the recognition of self- and non-self antigens.10,11,12 During this process, T cell effector functions are suppressed via signals generated by IRs such as cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), programmed cell death-1 (PD-1), lymphocyte activation gene-3, (LAG-3), and T cell immunoglobulin mucin-3 (TIM-3).13 This gradual loss of T cell effector function, especially experienced due to continued antigen exposure, has led to these cells being referred to as “exhausted.”13 Correspondingly, malignant tumors that express excessive inhibitory ligands, e.g., PD-L1, within the tumor and in its vicinity are more sensitive to treatment with antibodies toward IR. A side effect of these treatments is that patients develop signs of loss of self-tolerance, which may lead to autoimmune diseases.13,14 In addition, an increased PD-1 activity has been associated with decreased progression of erosions in rheumatoid arthritis (RA). In accordance with these findings, it has been proposed that blockade of PD-1 in malignancies could lead to osteoporosis.15,16 Recent studies of subsets of fibroblast-like synoviocytes (FLSs) have shown that these also have a proinflammatory and erosive phenotype. This phenotype is characterized by the expression of PDPN+THY1+ and associated with persistent inflammation in RA.17,18
With regard to children with inflammatory disease, little is known of their immunological response to the use of IRs. In the present study. we used an ex vivo model using synovial cells, which is commonly applied in RA.19,20 This ex vivo model enabled us to examine the effects of IRs in JIA. We report that although IRs are increased in patients with oligoarticular JIA (o-JIA), they show no signs of being exhausted. Our results also suggest that LAG-3 seems to be a contributing factor for governing continued the production of effector molecules in this disease.
Materials and methods
Twenty-four o-JIA patients were included from the Department of Pediatric Rheumatology, Hacettepe University Faculty of Medicine, Ankara, Turkey. All o-JIA patients were classified according to the ILAR criteria.2 SF and venous blood samples were obtained from patients referred for an intraarticular injection with corticosteroids. Fluids were immediately transferred to EDTA tubes and processed within 30–45 min. Mononuclear cells were separated by Ficoll-based density gradient method and aliquoted as peripheral blood mononuclear cells (PBMCs) and SF mononuclear cells (SFMCs), stored at −150 °C. Plasma samples were stored at −80 °C. Paired plasma and SF from five non-arthritic juvenile orthopedic controls (OCs) obtained during arthroscopy for ligament injury and plasma from 14 age- and sex-matched healthy controls (HCs) without signs of immune system activation were also analyzed. The samples were collected at Hacettepe University, Ankara, Turkey and transferred to DeleuranLab, Institute of Biomedicine Aarhus University, Aarhus, Denmark, where all the experiments were performed. The acquisition, storage, and international sharing of samples had been approved by the Hacettepe University Ethics Committee for Non-Interventional Clinical Trials (GO 18/277-35), and informed written consent was obtained from the parents and patients. Clinical features of the patients (including demographics, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), 27-joint Juvenile Disease Activity Score (JADAS-27), and hemogram) at the time of sample collection were also recorded.
Ex vivo arthritis model experiments
SFMCs from o-JIA patients (n = 6) were thawed and cultured in Dulbecco’s modified Eagle’s medium supplemented with fetal calf serum 10%, penicillin/streptomycin 1%, HEPES 1%, and glutamine 1% at a density of 1 × 106 cells/ml at 37 °C in a humidified incubator with 5% CO2. The culture medium was changed every 3–4 days. Differentiated fibroblasts (FLSs) were collected and stored at −80 °C after a median of 46 days of culture (passage 3 or 4) in the stimulation assay.
FLSs were thawed and seeded into 48-well plates (20,000–30,000 cells/well) 24 h before the experiment to let them attach to the plate surface. After 24 h, when the fibroblasts reached 70% confluency, autologous SFMCs and PBMCs (100,000–150,000 cells/well) were co-cultured with these FLSs. The following co-cultures were included in the possible representatives of the ex vivo arthritis models (FLSs only, SFMCs only, PBMCs only, FLSs + PBMCs, and FLSs + SFMCs) for o-JIA. These were incubated with 10 μg/ml neutralizing antibodies against the following IRs: PD-1 (Pembrolizumab), CTLA-4 (Ipilimumab), LAG-3 (clone 161012, BPS Bioscience), and TIM-3 (clone F38-2E2, BioLegend), and isotype controls for 48 h. For positive control, stimulatory anti-CD3/CD28 antibodies (Bead/cell ratio was 1:2; Dynabeads Human T activator CD3/CD28, Gibco) were used. In a similar setup, FLSs were thawed and seeded into 48-well plates (20,000–30,000 cells/well) 24 h before the experiment to let them attach to the plate surface. After 24 h, when the fibroblasts reached 70% confluency, PBMCs (100,000–150,000 cells/well) were co-cultured with them. FLS + PBMC co-cultures were incubated with 0.5 μg/ml agonist LAG-3 antibody (IMP761, donated by Frederic Triebel, Immutep) and control for 48 h (n = 5). After 48 h, supernatants were frozen at −20 °C and FLSs were collected by trypsinization and stored at −150 °C for further analysis.
Enzyme-linked immunosorbent assay (ELISA)
Quantifications of plasma and SF levels of soluble CTLA-4, PD-1, LAG-3, and TIM-3 were performed by using a sandwich ELISA. Soluble levels of co-IRs were determined in accordance with the manufacturer’s instructions using commercially available kits (PD-1 and TIM-3, R&D Systems; LAG-3 and CTLA-4, eBioscience). Samples were supplemented with heat-inactivated normal mouse, goat, and bovine IgG (Jackson ImmunoResearch Europe Ltd) to ensure pre-aggregation of heterophilic antibodies in the samples examined.21 Supernatants from ex vivo disease models were analyzed for monocyte chemoattractant protein-1 (MCP-1, detection limit 2.3 pg/ml, BioLegend), interferon-gamma (IFN-γ, detection limit 4 pg/ml, BioLegend) and interleukin-2 (IL-2) (Thermo Fisher, detection limit 2 pg/ml). Samples were analyzed in duplicate using the average of the optical density values to calculate concentrations.
Mesoscale V-plex
PBMCs and PBMCs + FLSs were cultured with the addition of a LAG-3 agonistic (IMP761) and control, both generously donated by Immutep. Supernatants from the described above cell cultures were examined for IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, and TNF-α by V-plex (Meso Scale Discovery®, V-PLEX® Proinflammatory Panel 1 (human)) according to the manufacturer’s guidelines.
Flow cytometry
PBMCs from EDTA whole blood and SFMCs from SF samples from o-JIA patients (n = 11) were isolated using Ficoll-Paque PLUS (GE Healthcare, Waukesha, WI). Staining focusing on T cells was performed with CD4 BV605 (clone RPA-T4, BioLegend), CD3 FITC (clone UCHT-1, BioLegend), and CD45RO APC (clone UCHL-1, BioLegend), excluding dead cells with Live/Dead near IR staining (clone L10119, Thermo Fisher Scientific). Staining focusing on the surface expression of IRs on T cells was examined with PD-1 Alexa Flour 405 (clone 913429, R&D System), TIM-3 BV510 (clone F38-2E2, BioLegend), CTLA-4 PE-Cy7 (clone L3D10, BioLegend), and LAG-3 PE-eFlour 610 (clone 3DS223H, Thermo Fisher Scientific). In all the panels, antibodies were titrated to the optimal working concentration. Gating was done on lymphocytes, excluding doublets and dead cells. Gates were set using FMOs. CD3+CD4+CD45RO+ cells were investigated for their expression of T cell IRs.
FLSs from ex vivo disease model (n = 4) were collected after 48 h by trypsinization and analyzed for MHC class II expression. For this purpose, cells were stained with CD45-PerCP (clone 2D1, BD Bioscience), CD45-BV650 (clone HI30, BioLegend), podoplanin-PE (clone NZ-1.3, Invitrogen), Thy1 (CD90)-PE-Cy7 (clone 5E10, BD Pharmingen), and HLA-DR-APC (clone L243, BD Bioscience). Gating was done on CD45 cells, excluding doublets and dead cells. Gates were set using FMOs. All samples were analyzed within 24 h using the Novocyte 3000 Cell Analyzer. Data analyses were performed using the FlowJo for Mac software (version 10.1) (Tree Star Inc., Ashland, OR).
Statistical analysis
Data were analyzed using GraphPad Statistics for MAC (version 6.0). Descriptive statistics of baseline characteristics were summarized using median and interquartile range (IQR) for numeric variables and percentages for categorical variables. Differences in soluble levels of IRs and expression of surface receptors were tested with the Mann–Whitney U test; MCP-1, IFN-γ, and IL-2 level fold changes were tested by Kruskal–Wallis analysis of variance. Post hoc tests were performed to explore significant differences between pairs, and p values were adjusted by using Bonferroni’s correction for multiple comparisons. Correlations between soluble levels and the surface expressions of IRs were analyzed by Spearman’s rank correlation coefficient test. A p value of 0.05 was considered statistically significant.
Results
Clinical and laboratory characteristics
o-JIA patients, HCs, and OCs were included in the study group. Details are presented in Table 1. In short, the median age of the o-JIA patients was 11.2 (6.7–14.2) years at the time of inclusion; they had a median JADAS-27 score of 7.4 (5.2–9.6) and a median disease onset of 3.6 (2.4–8.6) years. The age of the HCs was 9.0 (7.3–11.0) years and that of the OCs was 14.0 (14.0–17.5) years, expressed as median (IQR). Of the o-JIA patients, 12 received treatment with NSAIDs, 6 methotrexate, 1 adalimumab, and 1 etanercept.
Oligo-JIA patients (n = 24) were enrolled at a time of a flare with at least one swollen joint from which joint fluid could be drawn. Most of them were off-treatment (n = 16) at the time of sampling, while six received methotrexate treatment for <3 months and two patients were treated with anti-TNF agents for <6 months.
Increased levels of soluble IRs in JIA
The levels of the soluble IRs, PD-1, LAG-3, and TIM-3, were all significantly increased in the SF compared with plasma in o-JIA patients (Fig. 1). Whereas only sTIM-3 was significantly increased in SF compared with the plasma in OCs (p < 0.01). Furthermore, plasma sLAG-3 (p < 0.05) levels in o-JIA patients were significantly higher than the plasma levels in HCs. (Fig. 1) Soluble CTLA-4 was in all cases below the detection limit of 0.13 ng/ml in both plasma and SF.
a Soluble PD-1 levels of JIA SF were higher than JIA plasma. b Soluble LAG-3 levels of JIA SF were higher than JIA plasma and OC SF. JIA plasma levels were higher than HC plasma and OC plasma. c Soluble TIM-3 levels of JIA SF were higher than JIA plasma and OC SF (JIA patients n = 24, HC n = 14, OC n = 5). HC healthy controls, OC non-arthritic juvenile orthopedic controls, JIA juvenile idiopathic arthritis, SF synovial fluid, sPD-1 soluble programmed death-1, sLAG-3 soluble lymphocyte activation gene-3, sTIM-3 soluble T cell immunoglobulin mucin-3. Boxes indicate median and IQR, and whiskers indicate 10h–90th percentiles. *P < 0.05; **p < 0.01; ***p < 0.001.
There was a significant correlation between the plasma and SF levels of sPD-1 (r = 0.65, p = 0.029) and similarly the plasma and SF levels of LAG-3 (r = 0.51, p = 0.037) in o-JIA patients; however, no such correlation was observed for plasma and SF levels of sTIM-3. Furthermore, plasma sTIM-3 levels were intercorrelated with plasma sLAG-3 (r = 0.51, p = 0.039) levels.
None of the soluble IRs levels correlated with the clinical and laboratory parameters included (JADAS-27, CRP, ESR, and WBC).
The inflamed joint is the major site for the cellular expression of IRs in JIA
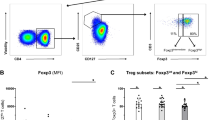
Memory-prone T-helper cells (CD3+CD4+CD45RO+) were investigated for their expression of IRs. The ratio of CD3+CD4+ helper T cells among lymphocytes was similar in PBMCs and SFMCs. However, as expected, the ratio of CD3+CD4+CD45RO+ T cells was significantly higher in SF, in accordance with increased antigen presentation within the diseased joint (Fig. 2a). Expression of all the examined IRs was detected in both the blood and SF cells. As observed for the soluble factors, IRs expressing CD3+CD4+CD45RO+ T cells were highly increased in the SF, compared with the blood (Fig. 2b–d). Similar to sCTLA-4, the cells expressing CTLA-4 were not increased in o-JIA SF.
a Gating strategy of CD3+ and CD4+ in total lymphocytes, CD45RO+ in CD3+CD4+ T cells, counter plot, and median percentages in the parent population. b Gating strategy of cells expressing IRs on CD3+CD4+CD45RO+ T cells in PBMC and SFMC. c The percentage of CD3+CD4+CD45RO+ cells expressing LAG-3 and TIM-3 and their MFIs were increased in the SFMC. PBMC peripheral blood mononuclear cells, SFMC synovial fluid mononuclear cells, MFI median fluorescence intensity, CTLA-4 cytotoxic T-lymphocyte-associated antigen-4, PD-1 programmed death-1, LAG-3 lymphocyte activation gene-3, TIM-3 T cell immunoglobulin mucin-3. Boxes indicate medians and IQRs, and whiskers indicate 10–90th percentiles. *P < 0.05; ***p < 0.001.
The surface expressions of PD-1 and TIM-3 on PBMCs were closely correlated (r = 0.71; p = 0.01). However, we did not observe any other correlations between the surface expression of the IRs on PBMCs and their soluble blood plasma levels. This was also the case with their surface expression on SFMCs and soluble SF levels.
Neutralization of LAG-3 increases MCP-1 secretion
To examine the role of FLSs in o-JIA, we established cultures from the o-JIA SFMCs, and here, >94% belonged to the PDPN + THY1 + subset (data not shown). This specific FLS subgroup, co-expressing podoplanin (PDPN) and CD90 (THY1), was shown to be associated with the disease activity in RA in the recent studies.17,18 As CD4 T cell exhaustion is an outcome of continued antigen presentation, we examined whether MHC class II expression was present on these FLSs. FLSs expressed almost no MHC class II molecules in monocultures, but after culturing these with autologous PBMCs and SFMCs, MHC class II was readily detected (Fig. 3). We used MCP-1 secretion as a reporter cytokine in this ex vivo disease model. MCP-1 levels were significantly higher in the autologous co-cultures, compared with monocultures. A synergistic effect was observed in the autologous co-cultures, rather than an additive effect, which supports that FLSs and PBMCs/SFMCs interact with each other when cultured together to become more proinflammatory (Fig. 4a).
a MHC class II expressions on CD45-PDPN + FLS increased when these were co-cultured with autologous SFMC (boxes indicate medians and IQRs). *P < 0.05. b Surface expression of MHC class II on CD45-PDPN + FLS, a representative sample from (a), counter plot. MHC major histocompatibility complex, PDPN podoplanin, FLS fibroblast-like synoviocytes, PBMC peripheral blood mononuclear cells, SFMC synovial fluid mononuclear cells.
a Spontaneous production of MCP-1 in ex vivo arthritis model (***p < 0.001). b Addition of neutralizing antibodies against CTLA-4, PD-1, LAG-3, and TIM-3 showed only a significant increase in MCP-1 production when LAG-3 was neutralized in PBMC and PBMC co-cultured with FLS, expressed as mean ± SEM (pg/ml) on the graph. The inserted heatmap shows the mean fold change to isotype control (*p < 0.05). FLS fibroblast-like synoviocytes, PBMC peripheral blood mononuclear cells, SFMC synovial fluid mononuclear cell, MCP-1 monocyte chemoattractant protein-1, CTLA-4 cytotoxic T-lymphocyte-associated antigen-4, PD-1 programmed death-1, LAG-3 lymphocyte activation gene-3, TIM-3 T cell immunoglobulin mucin-3.
To evaluate the degree of IRs’ ability to influence inflammation in this model of JIA, we added neutralizing antibodies toward CTLA-4, PD-1, LAG-3, and TIM-3 to the cultures. Only addition of neutralizing anti-LAG-3 antibodies significantly increased the MCP-1 levels of both PBMC monocultures (p < 0.01) and FLS + PBMC co-cultures (p < 0.01, Fig. 4b).
Agonistic activation of LAG-3 decreases cytokine production in o-JIA
Approaching the clinical setting, we added an agonistic LAG-3 antibody (IMP761) to o-JIA co-cultures and measured the diversity of cytokines (n = 5). As the highest MCP-1 production was seen in co-cultures with FLSs and PBMCs, both spontaneously and after blocking LAG-3, we used this setup to test the efficacy of IMP761. The addition of IMP761 (0.5 µg/ml) resulted in a decreased secretion of nearly all measured cytokines, and that of IL-10, IL-12, IL-1β, IL-4, and IL-6 reached the level of significance (p < 0.01, Fig. 5).
Discussion
It is well established that the adaptive immune system is central for disease pathogenesis in JIA by perpetuating inflammation of the diseased joint, which subsequently leads to cartilage and bone destruction. While IRs play an important immunoregulatory role in optimizing our adaptive immune response, little is known of their role in children and even less in different inflammatory conditions like o-JIA. In this study, we are the first to show that children with o-JIA have a skewed LAG-3 metabolism and suggest that they could benefit from agonistic LAG-3 activity.
We could not show any correlation between IR levels and laboratory parameters or disease activity scores. In o-JIA patients, we usually do not see high ESR or CRP values; they are generally mildly elevated. Thus, JADAS-27 scores were not very high and it was pretty much the same in most of our patients. The smoldering disease set or the low disease activity may have hindered a significant change in the IR levels.
The lack of animal models for JIA may be one of the reasons for the scarcity of new treatment options in this disease. We, therefore, used an ex vivo model of cultured synovial cells from o-JIA patients. In adults, this is a well-established model that utilizes cells taken from the site of pathology and is driven by the sum of immunological factors, including antigen presentation.19,20 This is further supported in our setup because we used an antibody that only detects the expression of intact assembled MHC class II molecules.22 Besides CD4, LAG-3 has also been reported to be expressed by CD8 T cells, B cells, and plasmacytoid cells, but its function in these cells is not known in detail.23,24,25 However, there are evidences to suggest that LAG-3 could play a role in diseases that are not commonly associated with CD4–MHC-II interactions. This is, to some extent, supported by a recent study in which LAG-3 was implicated in the outcome of psoriasis.26
Furthermore, a recent study showed that PD-1 expressing CD8+ T cells from JIA patients were not in a state of “exhaustion.”27 This is in agreement with the observation that JIA patients have a continued production of diverse effector cytokines and a normal vaccination response.28 Similarly, we observed that in spite of a high expression of IRs, PBMCs and SFMCs from o-JIA patients preserved their ability to produce cytokines in large amounts when activated through the CD3/CD28 pathway. On the other hand, in adult inflammatory arthritis, CD4+ T cells show distinct levels of exhaustion that can be reversed by anti-PD-1 antibody treatment.29 This suggests that o-JIA utilizes other pathways in the adaptive immune system than those observed in adult RA, even though both diseases seem to share similarities in their synovial phenotype. This is further supported by our measurements of soluble co-inhibitory factors. The plasma samples from HCs and OCs that were included showed a high concentration of sPD-1, whereas sCTLA-4 was below the detection limits. This is in contrast to what is observed in adult RA patients, in whom soluble forms of these molecules dominate.15,29 This finding does, however, indicate the severe degree of inflammation presented by o-JIA patients.
We added neutralizing antibodies against CTLA-4, PD-1, LAG-3, and TIM-3 to the cultures. These antibodies might neutralize the soluble IRs and have suppressive effects on membrane-bound IRs. In our co-culture setups, we, therefore, washed the cells and added new media together with the neutralizing antibodies. We, thus, find it unlikely that the soluble factors play a major role, as the neutralizing antibodies will quickly bind to membrane-bound receptors. We showed that LAG-3 plays a leading role in controlling the inflammation in children with o-JIA, and was superior to neutralizing PD-1, CTLA-4, and TIM-3. From a clinical perspective, the addition of an agonistic antibody resulted in a decrease in a broad spectrum of effector cytokines in just 48 h. LAG-3 agonists led to a general decrease in cytokines produced by activated T cells along with the levels of some anti-inflammatory cytokines such as IL-4 and IL-10. It is tempting to assume that the effects of LAG-3 are directed through inhibiting antigen presentation to memory-prone T-helper cells since these have a high expression of LAG-3, and LAG-3 competes with CD4 binding to MHC-II. LAG-3 is, however, also expressed by a T-regulatory cell (Treg).30 The Treg repertoire is highly restricted in both peripheral blood and SF in JIA patients.31 Tregs have been reported to have decreased function in both JIA and adult inflammatory arthritis.32 We, therefore, cannot rule out that some of the effects we observe by adding neutralizing antibodies can be due to further inhibition of Treg function. This does, however, further support the use of agonistic LAG-3 antibody treatment in o-JIA patients.
The clinical importance of perpetuating the activity of IRs in JIA is well established, as this has been described for CTLA-4 in the clinical setup.33,34 It was, therefore, intriguing that we did not see any effects of interfering with CTLA-4 in our study. Maggi et al. reported that CTLA-4 analog abatacept in vitro inhibits proliferation and cytokine production quickly after drug administration, but the clinical effect and reduction of inflammatory parameters in JIA patients was seen in 60–90 days.35 Similarly, when treating JIA with the CTLA-4 analog abatacept, the JADAS-71-CRP took up to 6 months to reach low disease activity, and patients continued to improve for up to 24 months after treatment initiation.33,34 Thus, the efficacy of agonistic LAG-3 treatment within 48 h in our setup suggests a possible additional treatment strategy in children with JIA.
This study has some limitations. We have investigated a relatively small number of patients. We did not analyze all T cell subtypes, but rather focused on the CD3+CD4+CD45RO+ population, which may make our results less solid. We observed a great individual variation regarding how a single individual with o-JIA reacts and expresses specific IRs. This is in full accordance with a recently published paper that reports the same observation in scleroderma.36 Furthermore, it is also in line with the experience in oncology. Many cancer patients experience a great response to the inhibition of IRs, but an even larger number of patients experience partial or no clinical benefit when given the same treatment.37,38 This confirms the individual patterns of IRs. It could also indicate that the redundancy within the system is high, presumably because of the importance of this system, as loss of function could potentially lead to an increased risk of developing autoimmunity.
Conclusion
This is the first report comparing the effects of different IRs in o-JIA. Our study revealed that both the soluble levels and the surface expressions of the co-IRs are increased at the site of inflammation in o-JIA. Co-cultures of autologous FLSs and PBMCs/SFMCs may serve as an important ex vivo arthritis model addressing also the stromal compartment recently discovered to be important in autoimmunity. The findings of our study support LAG-3 as a potential target in o-JIA. However, further in vivo studies are necessary to validate its role in JIA.
Data availability
All data generated or analyzed during this study are included in this paper and in its Supplementary information files.
References
Prakken, B., Albani, S. & Martini, A. Juvenile idiopathic arthritis. Lancet 377, 2138–2149 (2011).
Petty, R. E. et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J. Rheumatol. 31, 390–392 (2004).
Ozen, S. et al. Prevalence of juvenile chronic arthritis and familial Mediterranean fever in Turkey: a field study. J. Rheumatol. 25, 2445–2449 (1998).
Cimaz, R., Marino, A. & Martini, A. How I treat juvenile idiopathic arthritis: a state of the art review. Autoimmun. Rev. 16, 1008–1015 (2017).
Hinks, A. et al. Fine-mapping the MHC locus in juvenile idiopathic arthritis (JIA) reveals genetic heterogeneity corresponding to distinct adult inflammatory arthritic diseases. Ann. Rheum. Dis. 76, 765–772 (2017).
McIntosh, L. A. et al. Genome-wide association meta-analysis reveals novel juvenile idiopathic arthritis susceptibility loci. Arthritis Rheumatol. 69, 2222–2232 (2017).
Hinks, A. et al. Dense genotyping of immune-related disease regions identifies 14 new susceptibility loci for juvenile idiopathic arthritis. Nat. Genet. 45, 664–669 (2013).
Amariglio, N. et al. T-cell compartment in synovial fluid of pediatric patients with JIA correlates with disease phenotype. J. Clin. Immunol. 31, 1021–1028 (2011).
Wedderburn, L. R., Patel, A., Varsani, H. & Woo, P. Divergence in the degree of clonal expansions in inflammatory T cell subpopulations mirrors HLA-associated risk alleles in genetically and clinically distinct subtypes of childhood arthritis. Int. Immunol. 13, 1541–1550 (2001).
Nussing, S., Trapani, J. A. & Parish, I. A. Revisiting T cell tolerance as a checkpoint target for cancer immunotherapy. Front. Immunol. 11, 589641 (2020).
Zhang, Q. & Vignali, D. A. Co-stimulatory and co-inhibitory pathways in autoimmunity. Immunity 44, 1034–1051 (2016).
Schnell, A., Bod, L., Madi, A. & Kuchroo, V. K. The yin and yang of co-inhibitory receptors: toward anti-tumor immunity without autoimmunity. Cell Res. 30, 285–299 (2020).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
McKinney, E. F. & Smith, K. G. C. Metabolic exhaustion in infection, cancer and autoimmunity. Nat. Immunol. 19, 213–221 (2018).
Greisen, S. R. et al. Increased soluble programmed death-1 (sPD-1) is associated with disease activity and radiographic progression in early rheumatoid arthritis. Scand. J. Rheumatol. 43, 101–108 (2014).
Moseley, K. F. et al. Immune-related adverse events with immune checkpoint inhibitors affecting the skeleton: a seminal case series. J. Immunother. Cancer 6, 104 (2018).
Mizoguchi, F. et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 9, 789 (2018).
Dakin, S. G. et al. Pathogenic stromal cells as therapeutic targets in joint inflammation. Nat. Rev. Rheumatol. 14, 714–726 (2018).
Greisen, S. R. et al. Spontaneous generation of functional osteoclasts from synovial fluid mononuclear cells as a model of inflammatory osteoclastogenesis. APMIS 123, 779–786 (2015).
Schmidt, E. M. et al. Selective blockade of tumor necrosis factor receptor I inhibits proinflammatory cytokine and chemokine production in human rheumatoid arthritis synovial membrane cell cultures. Arthritis Rheum. 65, 2262–2273 (2013).
Kragstrup, T. W., Vorup-Jensen, T., Deleuran, B. & Hvid, M. A simple set of validation steps identifies and removes false results in a sandwich enzyme-linked immunosorbent assay caused by anti-animal IgG antibodies in plasma from arthritis patients. SpringerPlus 2, 263 (2013).
Qu, D. & Green, M. Folding and assembly of a human MHC class II molecule in a cell-free system. DNA Cell Biol. 14, 741–751 (1995).
Huard, B., Gaulard, P., Faure, F., Hercend, T. & Triebel, F. Cellular expression and tissue distribution of the human LAG-3-encoded protein, an MHC class II ligand. Immunogenetics 39, 213–217 (1994).
Kisielow, M., Kisielow, J., Capoferri-Sollami, G. & Karjalainen, K. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur. J. Immunol. 35, 2081–2088 (2005).
Workman, C. J. et al. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J. Immunol. 182, 1885–1891 (2009).
Ellis, J. et al. Depletion of LAG-3(+) T cells translated to pharmacology and improvement in psoriasis disease activity: a Phase I Randomized Study of mAb GSK2831781. Clin. Pharmacol. Ther. 109, 1293–1303 (2021).
Petrelli, A. et al. PD-1+CD8+ T cells are clonally expanding effectors in human chronic inflammation. J. Clin. Invest. 128, 4669–4681 (2018).
Ronaghy, A. et al. Vaccination leads to an aberrant FOXP3 T-cell response in non-remitting juvenile idiopathic arthritis. Ann. Rheum. Dis. 70, 2037–2043 (2011).
Frenz, T. et al. CD4(+) T cells in patients with chronic inflammatory rheumatic disorders show distinct levels of exhaustion. J. Allergy Clin. Immunol. 138, 586–589 (2016).
Huang, C. T. et al. Role of LAG-3 in regulatory T cells. Immunity 21, 503–513 (2004).
Henderson, L. A. et al. Next-generation sequencing reveals restriction and clonotypic expansion of Treg cells in juvenile idiopathic arthritis. Arthritis Rheumatol. 68, 1758–1768 (2016).
Rosenzwajg, M. et al. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann. Rheum. Dis. 78, 209–217 (2019).
Ruperto, N. et al. Long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis Rheumatol. 62, 1792–1802 (2010).
Brunner, H. et al. Subcutaneous abatacept in patients with polyarticular-course juvenile idiopathic arthritis: results from a Phase III Open-Label Study. Arthritis Rheumatol. 70, 144–1154 (2018).
Maggi, L. et al. Immunosuppressive activity of abatacept on circulating T helper lymphocytes from juvenile idiopathic arthritis patients. Int. Arch. Allergy Immunol. 171, 45–53 (2016).
Fleury, M. et al. Increased expression and modulated regulatory activity of coinhibitory receptors PD-1, TIGIT, and TIM-3 in lymphocytes from patients with systemic sclerosis. Arthritis Rheumatol. 70, 566–577 (2018).
Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018).
Boutros, C. et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 13, 473–486 (2016).
Acknowledgements
We would like to thank Karin Skovgaard Sørensen for her technical assistance during this study; to Aarhus University FACS core facility for their advice on the flow cytometry analyses, to Bahar Guclu, the patient/parent research partner, for her contribution. LAG-3 agonist (IMP761) was generously provided by Immutep and Dr. Triebel, CSO and CMO—per clause 10.2 of the MTA. This study was supported by a research grant from FOREUM Foundation for Research in Rheumatology. This study was also supported by the Danish Rheumatoid Association, Aarhus University, The Scientific and Technological Research Council of Turkey (TUBITAK), and the Hacettepe University Scientific Research Unit.
Author information
Authors and Affiliations
Contributions
E.S., S.O., and B.D. designed the study, collected the data, contributed to the data interpretation, and wrote the manuscript; E.S., S.D., E.T., Y.B., and S.O. collected patients’ data and samples, contributed the data interpretation, revised the manuscript; E.S., M.A., M.A.N., C.S., M.H., S.G., and B.D. designed and performed the experiments, contributed the data interpretation, and revised the manuscript. All authors made substantial contributions to either the conception and design of this study or to the generation, analysis, and/or interpretation of data. They agree to be accountable for the integrity of the work herein. All authors reviewed the manuscript and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Written informed written consent for publication was obtained from the parents and patients.
Ethical approval and consent to participate
This study was approved by the Hacettepe University Ethics Committee for Non-Interventional Clinical Trials (GO 18/277-35), and informed written consent to participate in the study was obtained from the parents and patients.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sag, E., Demir, S., Aspari, M. et al. Juvenile idiopathic arthritis: lymphocyte activation gene-3 is a central immune receptor in children with oligoarticular subtypes. Pediatr Res 90, 744–751 (2021). https://doi.org/10.1038/s41390-021-01588-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01588-2
This article is cited by
-
Targeting immune checkpoints in juvenile idiopathic arthritis: accumulating evidence
Pediatric Research (2021)