Abstract
Background
The objective of this study is to test how certain signs and symptoms related to COVID-19 in children predict the positivity or negativity of the SARS-CoV-2 nasopharyngeal swab in children.
Methods
We review the data of children who were tested for SARS-CoV-2 for a suspected infection. We compared the clinical characteristics of the subjects who tested positive and negative, including the sensibility, positive and negative predictive value of different combination of signs and symptoms.
Results
Of all the suspected infected, 2596 tested negative (96.2%) and 103 tested positive (3.8%). The median age was 7.0 and 5.3 years for the positive and negative ones, respectively. The female to male ratio was ~1:1.3. Fever and respiratory symptoms were mostly reported. Most positive children had a prior exposure to SARS-CoV-2-infected subjects (59.2%). A total of 99.3% of patients without fever nor exposure to the virus proved negative to the SARS-CoV-2 test.
Conclusions
Our study suggests that a child without fever or contact with infected subjects is SARS-CoV-2 negative. If this were to be confirmed, many resources would be spared, with improved care of both COVID-19 and not COVID-19-affected children.
Impact
-
Key message: lack of fever and exposure to SARS-CoV-2-infected people highly predicts a negative results of the SARS-CoV-2 nasopharyngeal swab in the paediatric population.
-
Added value to the current literature: this is the first article to prove this point.
-
Impact: reduction of emergency department accesses of children with suspected SARS-CoV-2 infection; increased outpatient management of children with cough or other common respiratory symptoms of infancy; sparing of many human and material health resources.
Similar content being viewed by others
Introduction
By overloading the health facilities of all the countries affected before posing a threat to the patient’s health, the pandemic of COVID-19 has become a true logistic challenge and, secondly, a medical issue. Starting from the 8th of December 2019, a total of 69,808,588 confirmed cases of COVID-19 and 1,588,854 confirmed deaths have been documented across the world.1
Italy is now facing a second surge of the infection with more tests, personnel, devices, intensive care unit beds and knowledge of the disease than during the spring outbreak. Up to the 2nd of December 2020, the Italian demographics of the disease shows that children younger than 19 years account for 12% out of the 1,624,269 confirmed cases of COVID-19, namely 3.6% in the 0–9 years cohort and 8.4% in the 10–19 years cohort. Most of these were asymptomatic or had mild symptoms, just a few were admitted to hospital and only 12 died.2 Out of the 100 Italian children younger than 18 years of age with laboratory confirmed COVID-19, the Coronavirus Infection in Paediatric Emergency Departments (CONFIDENCE) study reported 79 children with mild disease or no symptoms, 19 with moderate disease and 2 with severe disease, according to the categories described by Dong et al.3 Only 38 of these were admitted for their signs and symptoms. None died.4 However, such clinical presentations are only putatively associated with COVID-19, in a population where little is known, and few symptoms are usually evident.
The aim of this study is to investigate how well can respiratory symptoms, fever and exposure to infected subjects, or a combination of those, predict the positivity of the real-time reverse-transcription polymerase chain reaction (RT-PCR) assay for Sars-CoV-2 in nasal or nasopharyngeal swabs.
Materials and methods
Study design and setting
For the purposes of our study, we reviewed the cases of 2940 children who underwent RT-PCR assay for Sars-CoV-2 in nasal or nasopharyngeal swabs, admitted to the Paediatric Emergency Department (ED) of the Bambino Gesù Children’s Hospital in Rome, Italy, between the 9th of September 2020 and the 31st of October 2020. The Bambino Gesù Children’s Hospital is a tertiary care children’s hospital located in Rome, region of Lazio, Italy. The ED provides free urgent medical care on a 24/7 basis. In 2020, there were 43,945 (7081 in the study period) ED visits, and 7689 (1065 in the study period) urgent inpatient admissions.
In the study period, ED flows were organized to separate children with fever and respiratory symptoms from other patients and infection prevention and control measures were enforced. In fact, all patients and one parent were actively screened when entering the hospital using a structured way checking for fever, respiratory symptoms, and possible contacts with COVID-19 cases. Pre-triage stands were set up and only one parent or relative per patient was allowed to enter the hospital.
In all subjects, the test was carried out for one of two reasons: either for a clinical suspicion of infection or to allow a “clean” admission to non-COVID-19 dedicated wards. Suspicion of infection with SARS-CoV-2 was based on a history of exposure to infected subjects and/or the presence of fever and any respiratory symptom, comprising cough, dyspnoea and/or rhinorrhea, both at the time of presentation or in the recent past. When the parents reported any of the given characteristics at the triage, the children followed a dedicated path, with all isolation procedures implemented.
Test characteristics
“STARMag 96 9 4 Universal Cartridge kit” (Seegene Inc., South Korea) was used for the extraction and isolation of nucleic acids from nasopharyngeal swabs and biopsy samples, followed by multiplex real-time RT-PCR assay for simultaneous detection of three target genes of SARS-CoV-2, RdRP and N genes specific for SARS-CoV-2, and E gene for all Sarbecovirus, including SARS-CoV-2 (“‘AllplexTM 2019-nCoV Assay”, Seegene Inc.).
The collection of respiratory secretions via nasopharyngeal swabs was preferred, as they are easier to perform and have a high positivity rate, lower than nasopharyngeal aspirates, but significantly higher than oropharyngeal swabs.5,6
Assay characteristics
Nasal o nasopharyngeal swabs have been analysed with three platforms, according to the patients’ clinical conditions.
Patients with less critical conditions were given the swab results within 2–5 h or within 12 h, according to their need for further testing and care within the hospital facilities.
Samples of acute patients in critical conditions or requiring fast admittance to the ward were analysed with the test Cepheid Xpert Xpress SARS-CoV, which gives results in <1 h.
Samples of acute patients in non-critical conditions were analysed with the test LIAISON® MDX, which gives the result in 2–5 h.
Finally, samples of non-acute patients were analysed with Starlet-Tanbead platform. Results were analysed automatically using Seegene software (Seegene SARS-CoV-2 viewer).
Samples were considered positive, when one or more genes (genes E and N for the “fast” test; genes S and ORF1ab for the “intermediate” test; genes E, RdRP/S and N for the “slow” test) were detected.
Study cohort
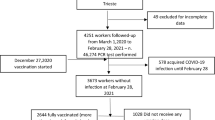
We divided all the eligible subjects in two groups (see Fig. 1).
Group A included those who were tested for a clinical suspicion of COVID-19, when presenting with any of the following: fever and/or respiratory symptoms and/or exposure to subjects with proven SARS-CoV-2 infection. Group B included all the subjects who required admission to the hospital for other reasons and were tested for hospital infection prevention protocol. We furtherly divided each group in two subgroups, according to the positive or negative result of the RT-PCR assay for Sars-CoV-2 in nasal or nasopharyngeal swabs, and we carried out a different analysis for each of them. We included all the negative and positive children of group A in the comparative analysis. The children who tested positive in group B were considered for a separate description (four cases). We excluded all the remaining subjects, namely those who tested negative in group B (241 subjects).
Data collection
Of all included subjects, we reported the presence or absence of the following clinical variables: fever, cough, dyspnoea, rhinorrhoea, sore throat or pharyngitis, vomiting, diarrhoea, abdominal pain, rash, headache, asthenia, or hypo-reactivity (in infants), conjunctivitis, anosmia or ageusia, a history of exposure to Sars-CoV-2-infected subjects, any coexisting condition and a history suggestive of a cause different from COVID-19 for the presenting symptoms. Symptoms such as headache, anosmia or ageusia, and thoracic pain were evaluated and recorded only in patients aged six or more. Fever was recorded if the child was febrile at presentation with a measured temperature of 37.8 °C or higher, or if the patient was not febrile at presentation, and the parents reported a measured temperature of 37.8 °C or higher up to 48 h before ED referral. All the other signs and symptoms were recorded whenever the parents reported them. A history of exposure to SARS-CoV-2-infected subjects was recorded only if direct contact occurred: all indirect or secondary contacts were excluded. We included both comorbidities and other pathologic conditions (such as a history of wheezing or recurrent infections) under the term “coexisting conditions”. Each of those could be either suggestive of the presenting symptoms or not, according to the history and clinical presentation. For instance, a child with febrile neutropenia following chemotherapy was classified as having a comorbidity (cancer) and a pathologic condition (neutropenia), which could explain the clinical presentation (fever). On the other hand, a patient with heart failure owing to a known cardiopathy, presenting with peripheral oedema primarily and cough collaterally, was classified as having a coexisting condition “not suggestive of symptoms”.
Data analyses
The software IBM SPSS version 23.0 was used for statistical analysis. All data are expressed as means, ranges and relative percentages. We compared the frequency of each clinical variable between the two subgroups of group A. Odds ratio and chi-squared tests were used for the comparison of proportions between the groups. A p value <0.05 was considered significant. Finally, we calculated the sensitivity, specificity, positive predictive value and negative predictive value of different combination of signs and symptoms, namely fever, respiratory symptoms, exposure to SARS-CoV-2-infected subjects and each of their combinations, via contingency tables. The current national prevalence of COVID-19 in the paediatric population was fitted into the analysis via likelihood ratios.
Results
The observed clinical variables and their derivatives are outlined in Table 1A, B and Table 2.
A total of 2940 subjects were tested during the selected time frame. A total of 2699 of these were tested for a clinical suspicion of infection (either in the form of fever, respiratory symptoms and/or exposure to subject with proven COVID-19), and 241 were tested as a safety procedure before being admitted to hospital for other reasons. We allocated the two samples to group A and B, respectively.
In group A, 2596 tested negative (96.2%), and 103 tested positive (3.8%).
The median age was slightly higher in the subgroups of those who tested positive (7.0 vs 5.3 years, p = 0.439). The age from 1 to 6 years was most represented in the negative subgroup (48.9%), whereas most subjects in the positive subgroup were aged 10 years or older (34.9%). There was a male predominance in both subgroups, with a female to male ratio of ~1:1.3. Most patients had no comorbidities or coexisting conditions before accessing the ED. However, there was a higher prevalence of comorbidities in the negative subgroup (16.4%), usually not correlated with the presenting symptoms (8.6%). In the positive subgroup, only a minority of cases presented with coexisting conditions or comorbidities (6.8%). In this subgroup, the respiratory symptoms could be attributed to such conditions or comorbidities in a handful of patients (5.8%). Interestingly, a proven exposure to SARS-CoV-2-infected associates was found in most children who tested positive (59.2%) and in a much lower percentage of those who tested negative (10.8%). In both subgroups more than half the subjects presented with a temperature ≥37.8 °C (53% vs 60.2% in the negative and positive subgroups, respectively). However, about a fourth in the positive subgroup (23.3%) to a fifth in the negative subgroup (18.3%) of the patients had no significant respiratory symptoms. These subjects were tested as they reported fever or respiratory symptoms in the previous days, which were not detected at the clinical examination. All the symptoms identified were present in equal proportions in the two subgroups, with a slightly higher (not significant) prevalence of respiratory symptoms in the negative subgroup. Among the other symptoms, vomiting, diarrhoea, abdominal pain and rash were more frequent in the negative subgroup, with headache being the single most frequent symptom in the positive subgroup.
Of 2940 children tested for SARS-CoV-2, 2699 were tested for a clinical suspicion of Covid-19 (group A) and 241 were tested for a “clean” admission to the ward (group B). In group A, 2596 positive and 103 negative children underwent a comparative analysis. In group B, 236 negative cases were excluded from the study and 4 positive cases were descripted separetely.
When adjusting for age groups, no significant differences were found between positive and negative subjects for fever and respiratory symptoms (see Table 1B). The difference in exposure to SARS-CoV-2 was significant in all subgroups. Comorbidities were significantly more reported by children aged 10 years or more (p = 0.028). However, only two positive subjects belonged to this subgroup. Headache was also significantly more reported by children aged 10 years or more, namely seven out of the nine children in the positive group who complained of this symptom.
The sensitivity, specificity, and positive and negative predictive values of different combination of fever, exposure to the virus and respiratory symptoms are reported in Table 2.
We considered the respiratory symptom “sore throat” for descriptive reasons, but we excluded it from the statistical analysis because of its subjectivity and heterogenous recognition by the single clinician.
As expected, the highest sensitivity was obtained when the patients had at least one sign or symptom correlated with COVID-19, compared to when none was present (see Table 2, item 11). The specificity varied with each test, reaching its highest value when fever and exposure to the virus were compared to having one of each or none (see Table 2, item 4). The positive predictive value was <6% in all tests, except two (see Table 2, items 1 and 4) where it would still not exceed 40%. Throughout all the tests we found >90% negative predictive values. The highest values were obtained when considering exposure against no exposure (98.2%), fever against no fever (97%), exposure and fever against exposure or fever or none (97.2%), exposure and/or fever against neither exposure nor fever (99.3%), exposure and/or respiratory symptoms against neither exposure nor respiratory symptoms (98.1%), exposure and/or fever, and/or respiratory symptoms against neither exposure nor fever nor respiratory symptoms (99.7%).
When fitting the prevalence of COVID-19 in the paediatric population in Italy, roughly 12% at the 2nd of December 2020,2 into these calculation, most negative predictive values dropped under 95% (see Table 2). In two cases, the negative predictive values remained above the 95% threshold (see Table 2, item 8 and 11).
In group B, 238 subjects tested negative (98.3%), and 4 tested positive (1.7%). Among these, two patients required treatment of severe trauma, one came in with thrombopenia and one was a month and 18 days old neonate with a reported temperature of 37.5 °C, who was admitted for precaution.
Discussion
Our results are coherent with those reported in the literature. In a large US report on 69,703 paediatric cases of COVID-19, fever, cough and shortness of breath were the most commonly presenting symptoms, accounting for more than half the cases in younger and older children alike.7 In a previous systematic review of 119 studies on children with COVID-19, fever, cough and rhinorrhoea were reported the most, followed with decreasing trend by myalgias, fatigue, sore throat, gastrointestinal symptoms, headache, decreased oral intake and rash.8 In a recent report by Otto et al.9 children under 6 years were most frequently tested, while subjects older than 12 years proved to be positive with a higher frequency across all groups. Fever, cough, congestion or rhinorrhea, and shortness of breath were the most reported symptoms in patients who tested positive, most of whom had a prior exposure to a SARS-CoV-2-infected subjects. A similar distribution of age groups and symptoms were observed in our cohort. In our study, headache was most frequently reported by positive subjects aged six or older (17.3%). Interestingly, the first retrospective study on the epidemiology of 2135 children with COVID-19, conducted in China and published in March 2020,3 reported a lower percentage of patients under 1 year of age (11.7% against our 29.1%) and a higher fraction of asymptomatic subjects (12.9% against our 23.3%) compared to our study.
However, the more recent literature on such topic is concordant: children have less severe symptoms and are more often asymptomatic or oligosymptomatic. Therefore, they are less often tested, with a consequent underestimate of the true numbers infected.10
When accounting for the sensitivity, specificity, positive predictive value and negative predictive value of different combinations of signs and symptoms, no single symptom or combination of such produced a satisfying sensitivity or positive predictive value. However, negative predictive values >90% were obtained in all cases. More specifically, 97% and 98.2% of the patients with no fever or previous exposure to infected subjects, respectively, were expected to have a negative SARS-CoV-2 test. However, 38 subjects without fever and 42 without a history of exposure to the virus would still prove positive (see Table 2, items 1 and 2). When combining the two, up to 99.3% of patients with neither fever nor a history of exposure to the virus were expected to have a negative SARS-CoV-2 test result, leaving out just seven subjects who still proved positive to the test (see Table 2, item 8). This result was just 0.4% points below the negative predictive value of the absence of fever, exposure to the virus and respiratory symptoms, that is the combination all patients are normally screened with at the triage. This last finding may be argued, as all patients should have had a positive such screening. In other terms, the 325 patients who presented with neither fever, nor exposure, nor respiratory symptoms (see Table 2, Item 11) should not have been included in group A at all. Of course, the patients were not addressed to the COVID-19 dedicated path solely when presenting with one of the three signs we indicated, namely fever, exposure and/or respiratory symptoms, but also when none of these were present, yet the patient reported to have had one in the past. This led to the inclusion of more patients in group A than those screened by our “narrower” diagnostic filter.
As the region where our study was conducted (Lazio) has been less affected than other territories in Italy,2 we fitted the national prevalence of COVID-19 in the paediatric population into our calculation, and we observed a drop of most observed negative predictive values under the threshold of 95% (see Table 2). However, the adjusted negative predictive value of the absence of both fever and exposure to SARS-CoV-2 (see Table 2, item 8), dropped from 99.3% to only 97.5%. This significant finding may extend the validity of our experience to other regions in Italy, where a higher rate of COVID-19 was experienced at the time of this study.
To our knowledge, only one study evaluated the predictors of positivity to SARS-CoV-2 testing in the paediatric age.11 In this paper, the authors reported a twofold increase in the odds of testing positive in older children and a higher obesity prevalence among the positive. Another old retrospective case–control study, involving 788 tested subjects of all ages, found a significant association between a positive nasopharyngeal swab and a previous contact, with an infected individual or a high temperature at presentation.12 In this cohort, shortness of breath, cough and gastrointestinal symptoms were also strongly associated with a positive SARS-CoV-2 test.
The high negative predictive value of the lack of exposure to SARS-CoV-2 seems to contradict the reported low rate of viral transmission between children. In a recent systematic review and meta-analysis of 14 contact-tracing studies, including children,13 the pooled odds ratio estimates of being a child with secondary infection compared with being an adult was 0.56 (95% CI, 0.37–0.85). We accounted for this discrepancy with the inherent literature by considering that the previous studies were conducted when the population screening was not yet available, thus missing many asymptomatic (mainly children) cases. However, many studies are proving that children are not significant drivers of the community transmission of COVID-19, especially in schools.14,15
Conclusions
Despite the low prevalence of the disease in the paediatric age, older children usually develop a clinically apparent form of COVID-19, characterized by unspecific symptoms, such as headache. It is understandable that all paediatricians are tempted to label all children with such presentation as infected by SARS-CoV-2 until proven otherwise. However, our study implies that a history of exposure to SARS-CoV-2 is the most predictive index of current infection, and that a child without fever or contact with infected subjects, even when presenting with mild respiratory symptoms, in most cases will prove negative to SARS-CoV-2 testing. A major upturn of this “diagnostic razor” is that all general practitioners (family paediatricians in Italy) may treat a simple cough, a sore throat or any isolated respiratory symptom, avoiding unnecessary referral to the laboratories or hospitals, where diagnostic testing takes place. In conclusion, if our evidence were to be confirmed by further multicentre studies, the many human and material resources that are currently used to isolate and treat the putative infected ones would be prioritized, leading to an improved care of both COVID-19- and not COVID-19-affected children.
References
World Health Organization. Coronavirus disease 2019 (COVID-19): situation report https://www.who.int/publications/m/item/weekly-epidemiological-update-8-december-2020 (2020).
Italian National Health Institute (Istituto Superiore di Sanità). Coronavirus epidemic: situation report https://www.epicentro.iss.it/coronavirus/, https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_2-dicembre-2020.pdf (2020).
Dong, Y. et al. Epidemiology of COVID-19 among children in China. Pediatrics 145, e20200702 (2020).
Parri, N., Lenge, M. & Buonsenso, D., Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group. Children with COVID-19 in pediatric emergency departments in Italy. N. Engl. J. Med. 383, 187–90 (2020).
Heald-Sargent, T. et al. Age-related differences in nasopharyngeal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) levels in patients with mild to moderate coronavirus disease 2019 (COVID-19). JAMA Pediatr. 174, 902–3 (2020).
Palmas, G. et al. Nasal swab as preferred clinical specimen for COVID-19 testing in children. Pediatr. Infect. Dis. J. 39, e267–e270 (2020).
Stokes, E. K. et al. Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb. Mortal. Wkly Rep. 69, 759–65 (2020).
Hoang, A. et al. COVID-19 in 7780 pediatric patients: a systematic review. EClinicalMedicine 24, 100433 (2020).
Otto, W. R. et al. The epidemiology of severe acute respiratory syndrome coronavirus 2 in a pediatric healthcare network in the United States. J. Pediatr. Infect. Dis. Soc. 9, 523–9 (2020).
Zimmermann, P. & Curtis, N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr. Infect. Dis. J. 39, 355–68 (2020).
Murillo-Zamora, E., Aguilar-Sollano, F., Delgado-Enciso, I. & Hernandez-Suarez, C. M. Predictors of laboratory-positive COVID-19 in children and teenagers. Public Health 189, 153–7 (2020).
Sun, Y. et al., National Centre for Infectious Diseases COVID-19 Outbreak Research Team. Epidemiological and Clinical Predictors of COVID-19. Clin. Infect. Dis. 71, 786–92 (2020).
Thomas, J. et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 25, e204573 (2020).
Lee, B. & Raszka, W. V. Jr COVID-19 transmission and children: the child is not to blame. Pediatrics 146, e2020004879 (2020).
Villani, A. et al. School in Italy: a safe place for children and adolescents. Ital. J. Pediatr. 47, 23 (2021).
Author information
Authors and Affiliations
Contributions
Conceptualized and designed the article: M.R., U.R., G.P., and M.P. Collected data: M.R., M.A., and A.T.. Analyzed the data: M.R. Verified the underlying data and statistical analysis: M.R., U.R., and G.P. Drafted and wrote the manuscript and tables: M.R. Provided laboratory expertise, contributed to acquisition, analysis and interpretation of data: S.R., L. Colagrossi, L.P., L. Coltella, and M.A. Revised the manuscript for important intellectual content: B.S., A.R., C.F.P., P.R., and A.V. Reviewed and accepted the final manuscript: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
The work was approved by our Ethics Committee. Patient consent was not required for this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roversi, M., Raucci, U., Pontrelli, G. et al. Diagnosis of COVID-19 in children guided by lack of fever and exposure to SARS-CoV-2. Pediatr Res 91, 1196–1202 (2022). https://doi.org/10.1038/s41390-021-01585-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01585-5