Abstract
Severe neonatal hyperbilirubinemia (SNH) is a serious condition that occurs worldwide. Timely recognition with bilirubin determination is key in the management of SNH. Visual assessment of jaundice is unreliable. Fortunately, transcutaneous bilirubin measurement for screening newborn infants is routinely available in many hospitals and outpatient settings. Despite a few limitations, the use of transcutaneous devices facilitates early recognition and appropriate management of neonatal jaundice. Unfortunately, however, advanced and often costly screening modalities are not accessible to everyone, while there is an urgent need for inexpensive yet accurate instruments to assess total serum bilirubin (TSB). In the near future, novel icterometers, and in particular optical bilirubin estimates obtained with a smartphone camera and processed with a smartphone application (app), seem promising methods for screening for SNH. If proven reliable, these methods may empower outpatient health workers as well as parents at home to detect jaundice using a simple portable device. Successful implementation of ubiquitous bilirubin screening may contribute substantially to the reduction of the worldwide burden of SNH. The benefits of non-invasive bilirubin screening notwithstanding, any bilirubin determination obtained through non-invasive screening must be confirmed by a diagnostic method before treatment.
Impact
-
Key message: Screening methods for neonatal hyperbilirubinemia facilitate early recognition and timely treatment of severe neonatal hyperbilirubinemia (SNH). Any bilirubin screening result obtained must be confirmed by a diagnostic method.
-
What does this article add to the existing literature? Data on optical bilirubin estimation are summarized. Niche research strategies for prevention of SNH are presented.
-
Impact: Transcutaneous screening for neonatal hyperbilirubinemia contributes to the prevention of SNH. A smartphone application with optical bilirubin estimation seems a promising low-cost screening method, especially in low-resource settings or at home.
Similar content being viewed by others
Introduction—the relevance of bilirubin measurements
Worldwide, unconjugated hyperbilirubinemia still threatens the health of many newborn infants. Approximately 80% of term newborn infants develop physiologic unconjugated hyperbilirubinemia, which is transient and benign in the vast majority of these infants.1 A small, but non-negligible proportion may develop severe neonatal hyperbilirubinemia (SNH). An estimated one million newborn infants worldwide suffer from SNH, necessitating intensive treatment to reduce the risk of death or kernicterus spectrum disorders (KSD).2 The incidence of SNH varies between 2 and 42 per 100,000 live-born infants in high-income countries (HIC) and depends, at least in part, on the predefined total serum bilirubin (TSB) concentration for this diagnosis.3 The estimated incidence of SNH is higher in low-income and middle-income countries (LMIC) where limited access to health care facilities and appropriate treatment threatens the health of thousands of newborn infants.4 The estimated contribution of SNH and/or Rhesus disease to KSD is 73 per 100,000 live births and the mortality rate is 119 per 100,000 live births in Eastern Europe, Latin America, sub-Saharan Africa, and Asia.2 These numbers are surpassed by a mortality rate for neonatal jaundice of 730 per 100,000 live births in India.5 Worldwide, 114,000 infants may die per year.2 These data underline the clinical relevance of screening methods for SNH identification. The primary purpose of screening is timely detection of newborns at risk of developing KSD, while diagnostic tests can provide the quantitative confirmation of the extent of jaundice.6
This article aims to describe different noninvasive screening methods to assess TSB in newborns that have either been used, that are currently available, or that are in the process of being developed. We pay special attention to the advantages and disadvantages of each method.
Screening methods—noninvasive total serum bilirubin assessments
During the last centuries, neonatal jaundice had already been recognized as a risk to the health of newborns.7 Nevertheless, it was not until 1969 that Kramer systematically correlated dermal zones of jaundice with actual TSB.8 A number of studies evaluated the accuracy and reliability of visual assessment of jaundice, a long-standing way of estimating the severity of hyperbilirubinemia.9,10,11,12 Correlation coefficients between visual assessment and TSB concentrations vary between 0.35 and 0.75. Riskin and colleagues analyzed the correlation between visual assessment and TSB concentrations in 1129 term and late preterm neonates. They concluded that visual assessment was an unreliable tool to detect SNH before discharge. Approximately 60% of newborn infants were misclassified, which resulted in inadequate follow-up.11 In 2009, Keren and colleagues published a prospective cohort study on 522 term and late preterm infants who had been graded by nurses using the 5-point Kramer scale to determine the maximum cephalocaudal extent of jaundice before discharge.12 They found that progression correlates poorly with TSB concentrations. This scale should therefore not be used by clinicians to estimate TSB levels. Dr. Maisels dismissed the efficacy of visual assessment by stating “Bilirubin eyes do not exist” (pers. communication, Jonxis lecture, May 2010, Groningen, the Netherlands). It is neither objective, nor reliable or accurate, and interobserver agreement is poor.9,10,11,12 Despite these limitations, in many countries visual assessment of jaundice is still the only method available to screen for SNH. More reliable methods for screening infants for SNH are required.
Icterometers
To facilitate visual examination and reduce interobserver variability, different screening instruments have been developed. Davidson and colleagues “applied a tongue depressor with considerable force” to the mucous membrane of the lower jaw and to the skin of the forehead or the chin. In this way, jaundice that was not apparent otherwise, became visible.9 In 1958, Allen described the use of polished Perspex for the early detection of jaundice.13 One of the first instruments to estimate whether a TSB concentration should be obtained was the so-called icterometer. An icterometer is a Perspex ruler with two to six different hues of yellow arranged in a stepwise gradient. The icterometer is pressed on the nose, forehead, or inside of the newborn’s lip. The resultant yellow color of the blanched skin is matched with a hue of yellow, each of which corresponds with a range of TSB levels. In 1954, the Ingram or Gosset icterometer was developed.14 Gosset and colleagues found a correlation between every hue of yellow and range of TSB concentration. Nevertheless, they acknowledged the limitations of the icterometers in terms of accuracy and lack of color standardization. Subsequently, other studies evaluated the use of icterometers,15,16 while clinical guidelines explicitly advocated against their use.17 Images of old icterometers and novel icterometers currently in use can be accessed at https://www.northamptongeneral.nhs.uk/About/OurHistory/Dr-Gosset/The-Gosset-Icterometer.aspx#ad-image-0, https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0183882#, and at https://pediatrics.aappublications.org/content/pediatrics/143/5/e20182039/F1.large.jpg.
Olusanya and colleagues described the diagnostic performance of the Bilistrip™, a two-color (dark yellow and light yellow) icterometer used by 2492 Nigerian mothers to assess the presence (dark yellow) or absence (light yellow) of SNH in their infants.18 The mean transcutaneous bilirubin (TcB) concentration for dark yellow, 171 µmol/L, was significantly higher than for light yellow, 104 µmol/L. The sensitivity and negative predictive value of the Bilistrip™ was high—95.8%—for neonates requiring phototherapy (PT). Only one infant out of 24 who needed PT was missed. In the USA and Bangladesh, a completely renewed icterometer, the Bili-ruler™, was tested recently in 790 neonates of younger than 28 days to identify jaundice.19 The Bili-ruler™ has six color strips that were developed by processing digital color photographs of newborn infants with different TSB levels. Bili-ruler™ readings on the nose showed the best correlation with TcB concentrations (r = 0.76) as well as with TSB (r = 0.78). Sensitivity and specificity for readings ≥3.5 for TcB ≥222 µmol/L and for TSB ≥ 188 µmol/L were 90 and 86%, versus 85 and 83%, respectively. Moreover, interrater reliability was high. The authors conclude that the Bili-ruler™ “may be used to improve referrals from community or peripheral health centers to higher-level facilities with capacity for bilirubin testing and/or PT”.
Transcutaneous bilirubinometry
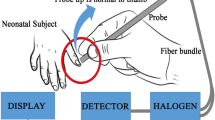
In 1978, both Hannemann and Peevy with their colleagues demonstrated a noninvasive method to determine TSB by spectral reflectance of neonatal skin.20,21 Two years later, Yamanouchi and colleagues described a linear relationship between the TSB concentration and the intensity of the yellowish skin color in terms and low birth weight infants.22 These authors correctly assumed that this correlation could ultimately provide the basis for a noninvasive monitoring device that could replace TSB measurements, at least in part. Currently, electronic transcutaneous bilirubin (TcB) devices are available to health care professionals for distinguishing clinically relevant SNH. During the last decades and mainly in industrialized countries that can afford such technology, TcB devices have become routine screening tools for neonatal jaundice. The advantage of this indirect screening method is that it is noninvasive, fast, easy to learn, and user friendly. The levels of TcB are estimated from the yellowish discoloration of the skin and subcutaneous tissue by measuring the difference in optical densities for a light of different wavelengths. Dark pigmentation of the skin may potentially confound light reflectance. Different methodologies have been developed by manufacturers of TcB instruments to minimize this effect.23,24 The American Agency of Pediatrics (AAP) guidelines suggest using TcB screening for neonatal jaundice prior to discharge but the use of TcB varies among nurseries, even within one country, from none to selective screening or routine, that is, universal screening.25,26 TcB values provide a reasonable estimate of TSB levels in healthy newborns with correlation coefficients ranging from 0.70 to 0.97.27,28,29,30 However, transcutaneous bilirubinometry does have its limitations for identifying SNH. The measuring scales of the TcB instruments are limited—no readings above 340 µmol/L. Moreover, these instruments may overestimate TSB at low values and underestimate TSB at high values.31 Aranda and colleagues showed that in various groups of term and preterm multi-racial sick neonates, TcB readings above 205 µmol/L should be confirmed with standard laboratory methods.32 Nevertheless, although TcB may underestimate TSB when concentrations are higher than 205 or 257 μmol/L, other authors report no increase of underestimation at a specific TSB level.33,34,35 The accuracy of TcB in infants receiving PT (due to skin bleaching) and its use on preterm neonates has also been subject of discussion.36,37 Hulzebos and colleagues concluded that PT enhances the well-known underestimation of TSB by TcB in preterm infants, even when measured on an unexposed area of skin in the diaper area. The underestimation of TSB before PT was significantly lower, 44 ± 36 μmol/L, than the underestimation during and after PT, 61 ± 29 and 63 ± 25 μmol/L, respectively.38 Other studies have highlighted discrepancies between TcB and TSB levels depending on ethnicity.39 Taylor and colleagues demonstrated that TcB measurements significantly overestimate TSB levels in African American newborns compared with newborns of other races.40 Similar results have been observed by Olusanya and Emokpae.41
The TcB values inform the health care professional about two questions: “Should I worry about this infant?” and “Should I obtain a TSB in this infant?”.42 To counter the shortcomings of transcutaneous bilirubinometry, various decision rules on TcB cut-off levels, that is when to obtain a TSB, have been proposed to minimize the risk of missing high TSB levels.43,44,45 One rule to correct for underestimation is to obtain a TSB when the TcB value is higher than the TSB of the PT threshold minus 50 μmol/L.46 The use of TcB and a recommended decision rule substantially reduces the need for TSB measurements in very preterm as in late preterm and term infants.26,33,38,45,46,47 In addition to single TcB measurements, the rate at which TcB values rise was found useful to identify late preterm infants at risk of severe hyperbilirubinemia.48,49 A few disadvantages of transcutaneous bilirubinometry notwithstanding,50 the advantages and proven reliability justify its use to screen for SNH, both in hospitals and in outpatient settings.
Smartphone or digital camera-based bilirubin estimation
A novel method to estimate TSB levels and to detect SNH is using the camera on a mobile phone in conjunction with advanced digital image processing techniques with red, green, and blue pixel color analyses and machine learning. The first proposals were posted online in 2012 and 2013, respectively.51,52 Given the widespread use of smartphones, even in LMICs, different smartphone applications (apps) were developed to facilitate neonatal jaundice screening. The apps use the yellowish discoloration of the newborn’s skin or whites of the eyes (sclerae).53,54,55,56,57,58,59,60,61,62,63,64,65,66 A color calibration card helps to attenuate variations in the lighting conditions of the surrounding environment and facilitates image capture and data extraction. By utilizing the calibration card various brands of smartphones with different lens qualities can be calibrated. A dermatoscope or magnification clip attached to the embedded smartphone camera may help to obtain detailed images. In studies that compare smartphone-based optical bilirubin estimates with TSB, correlation coefficients vary between 0.48 and 0.91 when optical bilirubin estimates were obtained from skin.53,55,56,57,58,59,60,61,64,66 They vary between 0.73–0.86 when the estimates were obtained from the sclerae.54,62,63,65 Although the sclerae are not influenced by skin pigmentation, mean differences between optical bilirubin estimates from skin or from sclerae and TSB, as well as diagnostic accuracy are comparable.58,65,66 Taylor and colleagues reported a mean (± SD) difference between their BiliCam bilirubin and TSB of 0.17 ± 31 µmol/L.58 Almost 92% of BiliCam bilirubin values were within 51 µmol/L of the paired TSB levels. The sensitivity was 100% with a specificity of 76.4% for identifying TSB ≥ 291 µmol/L. Aune and colleagues reported a mean (± SD) difference between the Picterus image estimate and TSB of − 0.2 ± 41 µmol/L. Sensitivity was 100% and specificity was 69% for identifying TSB > 250 μmol/L.66 In our opinion, this development heralds the beginning of a new era of research into affordable smartphone-based bilirubin estimation as a screening tool for SNH.
Future directions of research
As “no single intervention is likely to be sufficient” to prevent SNH and to reduce the incidence of acute bilirubin encephalopathy (ABE) and KSD, what would be the best scientific route to be taken to achieve this goal?67 Notwithstanding the ubiquitous need for low-cost bilirubin screening devices with high negative predictive values, Wennberg and colleagues of the Stop Kernicterus in Nigeria (SKIN) consortium demonstrated that maternal instruction on neonatal jaundice and timely care-seeking whenever jaundice was suspected, was associated with a reduction of ABE.68 As this points to the need for parental instruction on jaundice, it seems clear that future research on the prevention of SNH should encompass parental instruction, irrespective of the screening method used. In May 2021, the Better Assessment of Jaundice at home (BEATJaundice@home) study will start in the Netherlands. This study aims to test three screening methods for neonatal hyperbilirubinemia at home in 2000 neonates with a gestational age of at least 35 weeks. The screening methods are transcutaneous bilirubinometry (JM-105, Dräger, Lübeck, Germany), a commercially available point-of-care test for total bilirubin in whole blood (Bilistick®, Bilimetrix s.r.l., Trieste, Italy), and a smartphone application (Picterus, Trondheim, Norway). Another research niche will be the application of wireless wearable biosensors that allow for continuous noninvasive monitoring of transcutaneous bilirubin.69 While awaiting the results of BEATJaundice@home and other studies, we expect that in the near future research on artificial intelligence, machine learning technology, and biometrics may play an important role in early recognition of SNH and reduction of ABE and KSD.
Summary and conclusion
Any newborn infant may develop SNH. Timely detection and early treatment are essential to lower the worldwide burden of SNH. This implies that visual assessment of jaundice should be replaced by more reliable methods to assess TSB. The screening method should also be easy to perform, low-cost, safe, and have a high diagnostic accuracy. To minimize the risk of missing a newborn with (imminent) SNH, specific decision rules to account for inaccuracy of transcutaneous bilirubinometry are recommended. Indeed, TcB has replaced many invasive TSB measurements. Whether the same holds for other noninvasive bilirubin screening methods, that is, novel icterometers and smartphone-based optical bilirubin estimates, has yet to be determined. Whereas noninvasive bilirubin screening measurements are important in reducing the occurrence of SNH, invasive TSB measurements remain the gold standard and are essential for the definitive diagnosis of SNH and treatment decisions.
References
Bhutani, V. K. et al. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J. Pediatr. 162, 477–82.e1 (2013).
Bhutani, V. K. et al. Neonatal hyperbilirubinemia and Rhesus disease of the newborn: incidence and impairment estimates for 2010 at regional and global levels. Pediatr. Res. 74, 86–100 (2013).
Donneborg, M.L., Hansen, B.M., Vandborg, P.K., Rodrigo-Domingo, M., Ebbesen, F. Extreme neonatal hyperbilirubinemia and kernicterus spectrum disorder in Denmark during the years 2000–2015. J Perinatol. 40, 194–202 (2020).
Greco, C. et al. Neonatal jaundice in low- and middle-income countries: lessons and future directions from the 2015 Don Ostrow trieste yellow retreat. Neonatology 110, 172–80 (2016).
Bang, A. T., Bang, R. A., Baitule, S., Deshmukh, M. & Reddy, M. H. Burden of morbidities and the unmet need for health care in rural neonates – a prospective observational study in Gadchiroli, India. Indian Pediatr. 38, 952–65 (2001).
Ngashangva, L., Bachu, V. & Goswami, P. Development of new methods for determination of bilirubin. J. Pharm. Biomed. Anal. 162, 272–85 (2019).
Hansen, T. W. R. Pioneers in the scientific study of neonatal jaundice and kernicterus. Pediatrics 106, e15 (2000).
Kramer, L. I. Advancement of dermal Icterus in the jaundiced newborn. Am. J. Dis. Child 118, 454–8 (1969).
Davidson, L. T., Merritt, K. K. & Weech, A. A. Hyperbilirubinemia in the newborn. Am. J. Dis. Child 61, 958–80 (1941).
Moyer, V. A., Ahn, C. & Sneed, S. Accuracy of clinical judgment in neonatal jaundice. Arch. Pediatr. Adolesc. Med 154, 391–4 (2000).
Riskin, A., Tamir, A., Kugelman, A., Hemo, M. & Bader, D. Is visual assessment of jaundice reliable as a screening tool to detect SNH? J. Pediatr. 152, 782–7, e1–2 (2008).
Keren, R., Tremont, K., Luan, X. & Cnaan, A. Visual assessment of jaundice in term and late preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 94, F317–22 (2009).
Allen, F. H. Jr Early jaundice in the newborn; aids to detection. N. Engl. J. Med 258, 1302–3 (1958).
Gosset, I. H. A perspex icterometer for neonates. Lancet 1, 87–88 (1960).
Schumacher, R. E., Thornbery, J. M. & Gutcher, G. R. Transcutaneous bilirubinometry: a comparison of old and new methods. Pediatrics 76, 10–4 (1985).
Bilgen, H., Ince, Z., Ozek, E., Bekiroglu, N. & Ors, R. Transcutaneous measurement of hyperbilirubinaemia: comparison of the Minolta jaundice meter and the Ingram icterometer. Ann. Trop. Paediatr. 18, 325–8 (1998).
NICE National Institute for Health and Care Excellence. Neonatal jaundice 2010. https://www.nice.org.uk/guidance/cg98/evidence/full-guideline-245411821.
Olusanya, B. O., Slusher, T. M., Imosemi, D. O. & Emokpae, A. A. Maternal detection of neonatal jaundice during birth hospitalization using a novel two-color icterometer. PLoS ONE 12, e0183882 (2017).
Lee, A. C. et al. A novel icterometer for hyperbilirubinemia screening in low-resource settings. Pediatrics 143, e20182039 (2019).
Hannemann, R. E., DeWitt, D. P. & Wiechel, J. F. Neonatal serum bilirubin from skin reflectance. Pediatr. Res 12, 207–10 (1978).
Peevy, K. H. et al. Estimation of serum bilirubin by spectral reflectance of the skin, Duke University Medical Center. Pediatr. Res 12, 532 (1978).
Yamanouchi, I., Yamauchi, Y. & Igarashi, I. Transcutaneous bilirubinometry: preliminary studies of noninvasive transcutaneous bilirubin meter in the Okayama National Hospital. Pediatrics 65, 195–202 (1980).
https://www.draeger.com/en_me/Hospital/Products/Thermoregulation-and-Jaundice-Management/Jaundice-Management-and-Phototherapy/Jaundice-Screening/Jaundice-Meter-JM-105#instructions_for_use Last accessed at May 23rd, 2020.
https://philipsproductcontent.blob.core.windows.net/assets/20170616/f722df04b6ce4e7dad79a7940147347a.pdf Last accessed on May 23rd, 2020.
Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 114, 297–316 (2004).
Van den Esker-Jonker, B., Den Boer, L., Pepping, R. M. C. & Bekhof, J. Transcutaneous bilirubinometry in jaundiced neonates: a randomized controlled trial. Pediatrics 138, e20162414 (2016).
Chokemungmeepisarn, P., Tantiprabha, W., Kosarat, S. & Manopunya, S. Accuracy of the BilicareTM transcutaneous bilirubinometer as the predischarge screening tool for significant hyperbilirubinemia in healthy term and late preterm neonates. J. Matern Fetal Neonatal Med 33, 57–61 (2020).
Cucuy, M., Juster-Reicher, A., Flidel, O. & Shinwell, E. Correlation between transcutaneous and serum bilirubin in preterm infants before, during, and after phototherapy. J. Matern. Fetal Neonatal. Med. 31, 1323–6 (2018).
Arman, D., Topcuoğlu, S., Gürsoy, T., Ovalı, F. & Karatekin, G. The accuracy of transcutaneous bilirubinometry in preterm infants. J. Perinatol. 40, 212–8 (2020).
Nahar, N. et al. Comparison of Serum Bilirubin with Transcutaneous Bilirubinometry in Late Preterm and Term Newborn. Mymensingh Med. J. 26, 621–7 (2017).
Johnson, S. M. et al. Validation of transcutaneous bilirubinometry during phototherapy for detection and monitoring of neonatal jaundice in a low-income setting. Paediatr. Int. Child Health 40, 25–29 (2020).
Aranda Cazón, C., Torrubia Doredo, B., Álvarez López, C., De Gracia Hils, Y. & Cuadrado Cenzual, M. C. Determining the correlation and accuracy of three methods of measuring neonatal bilirubin concentration: serum, capillary and transcutaneous bilirubin. Biomed. J. Sci. Tech. Res. 1, 722–6 (2017).
Grohmann, K. et al. Bilirubin measurement for neonates: comparison of 9 frequently used methods. Pediatrics 117, 1174–83 (2006).
Hemmati, F. & Rad, N. A. K. The value of Bilicheck® as a screening tool for neonatal jaundice in the South of Iran. Iran. J. Med Sci. 38, 122–8 (2013).
Mussavi, M., Niknafs, P. & Bijari, B. Determining the correlation and accuracy of three methods of measuring neonatal bilirubin concentration. Iran. J. Pediatr. 23, 333–9 (2013).
Ozkan, H., Oren, H., Duman, N. & Duman, M. Dermal bilirubin kinetics during phototherapy in term neonates. Acta Paediatr. 92, 577–81 (2003).
Starowicz, O., Edwards, P., Schmidt, P. & Birch, P. Evaluation of the Kejian KJ-8000 bilirubinometer in an Australian setting. J. Paediatr. Child Health 56, 283–8 (2020).
Hulzebos, C. V., Vader-van Imhoff, D. E., Bos, A. F. & Dijk, P. H. Should transcutaneous bilirubin be measured in preterm infants receiving phototherapy? The relationship between transcutaneous and total serum bilirubin in preterm infants with and without phototherapy. PLoS ONE 14, e0218131 (2019).
Neocleous, C. et al. A comparison between transcutaneous and total serum bilirubin in healthy-term Greek neonates with clinical jaundice. Prague Med. Rep. 115, 33–42 (2014).
Taylor, J. A. et al. Discrepancies between transcutaneous and serum bilirubin measurements. Pediatrics 135, 224–31 (2015).
Olusanya, B. O. & Emokpae, A. A. Use of transcutaneous bilirubin to determine the need for phototherapy in resource-limited settings. Neonatology 111, 324–30 (2017).
Maisels, M. J. Transcutaneous bilirubin measurement: does it work in the real world? Pediatrics 135, 364–6 (2015).
Rodríguez-Capote, K., Kim, K., Paes, B., Turner, D. & Grey, V. Clinical implication of the difference between transcutaneous bilirubinometry and total serum bilirubin for the classification of newborns at risk of hyperbilirubinemia. Clin. Biochem. 42, 176–9 (2009).
Maisels, M. J., Bhutani, V. K., Bogen, D., Newman, T. B., Stark, A. R. & Watchko, J. F. Hyperbilirubinemia in the newborn infant > or = 35 weeks’ gestation: an update with clarifications. Pediatrics 124, 1193–8 (2009).
Maisels, M. J., Coffey, M. P. & Kring, E. Transcutaneous bilirubin levels in newborns <35 weeks’ gestation. J. Perinatol. 35, 739–44 (2015).
Nagar, G., Vandermeer, B., Campbell, S. & Kumar, M. Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics 132, 871–81 (2013).
Briscoe, L., Clark, S. & Yoxall, C. W. Can transcutaneous bilirubinometry reduce the need for blood tests in jaundiced full term babies? Arch. Dis. Child Fetal Neonatal Ed. 86, F190–2 (2002).
De Luca, D., Jackson, G. L., Tridente, A., Carnielli, V. P. & Engle, W. D. Transcutaneous bilirubin nomograms: a systematic review of population differences and analysis of bilirubin kinetics. Arch. Pediatr. Adolesc. Med 163, 1054–9 (2009).
Yu, Z. B., Han, S. P. & Chen, C. Bilirubin nomograms for identification of neonatal hyperbilirubinemia in healthy term and late-preterm infants: a systematic review and meta-analysis. World J. Pediatr. 10, 211–8 (2014).
Bosschaart, N. et al. Limitations and opportunities of transcutaneous bilirubin measurements. Pediatrics 129, 689–94 (2012).
Baker, C., et al. BME 272 NCIIA Project Proposal Neonatal Jaundice. (2012).
Patel, P. ClikJaundice: using mobile technology to detect yellow in newborns. http://thealternative.in/social-business/clickjaundiceusing-the-phone-to-prevent-jaundice-in-newborns/. (2013).
De Greef, L., et al. BiliCam: using mobile phones to monitor newborn jaundice. In: Proceedings of the 2014 ACM International Joint Conference on Pervasive and Ubiquitous Computing (UbiComp ’14); 331–342 (Seattle, WA, 2014).
Leung, T. S. et al. Screening neonatal jaundice based on the sclera color of the eye using digital photography. Biomed. Opt. Express 6, 4529–38 (2015).
Rong, Z. H. et al. Evaluation of an automatic image-based screening technique for neonatal hyperbilirubinemia. Zhonghua Er Ke Za Zhi 54, 597–600 (2016).
Aydin, M., Hardalac, F., Ural, B. & Karap, S. Neonatal jaundice detection system. J. Med. Syst. 40, 166 (2016).
Swarna, S., Pasupathy, S., Chinnasami, B., Manasa, D. N. & Ramraj, B. The smart phone study: assessing the reliability and accuracy of neonatal jaundice measurement using smart phone application. Int. J. Contemp. Pediatr. 5, 285–9 (2017).
Taylor, J. A. et al. Use of a smartphone app to assess neonatal jaundice. Pediatrics 140, e20170312 (2017).
Munkholm, S. B., Krøgholt, T., Ebbesen, F., Szecsi, P. B. & Kristensen, S. R. The smartphone camera as a potential method for transcutaneous bilirubin measurement. PLoS ONE 13, e0197938 (2018).
Leartveravat, S. Transcutaneous bilirubin measurement in full term neonate by digital camera. Med J Srisaket Surin Buriram Hospit. 24, 108–18 (2009).
Sufian, A. T., Jones, G. R., Shabeer, H. M., Elzagzoug, E. Y. & Spencer, J. W. Chromatic techniques for in vivo monitoring jaundice in neonate tissues. Physiol. Meas. 39, 095004 (2018).
Rizvi, M. R., Alaskar, F. M., Albaradie, R. S., Rizvi, N. F. & Al-Abdulwahab, K. A novel non-invasive technique of measuring bilirubin levels using BiliCapture. Oman Med J. 34, 26–33 (2019).
Leung, T. S., Outlaw, F., MacDonald, L. W. & Meek, J. Jaundice Eye Color Index (JECI): quantifying the yellowness of the sclera in jaundiced neonates with digital photography. Biomed. Opt. Express 10, 1250–6 (2019).
Padidar, P. et al. Detection of neonatal jaundice by using an android OS-based smartphone application. Iran. J. Pediatr. 29, e84397 (2019).
Outlaw, F. et al. Smartphone screening for neonatal jaundice via ambient-subtracted sclera chromaticity. PLoS ONE 15, e0216970 (2020).
Aune, A., Vartdal, G., Bergseng, H., Randeberg, L. L. & Darj, E. Bilirubin estimates from smartphone images of newborn infants’ skin correlated highly to serum bilirubin levels. Acta Paediatr. 1–7 (2020).
Watchko, J. F. Maternal instruction on neonatal jaundice: what can we learn from the Stop Kernicterus in Nigeria (SKIN) experience? J. Pediatr. 221, 7–8 (2020).
Wennberg, R. P. et al. Maternal instruction about jaundice and the incidence of acute bilirubin encephalopathy in Nigeria. J. Pediatr. 221, 47–54 (2020).
Inamori, G. et al. Neonatal wearable device for colorimetry-based real-time detection of jaundice with simultaneous sensing of vitals. Sci. Adv. 7, eabe3793 (2021).
Acknowledgements
We greatly appreciate the help of T. van Wulfften Palthe in correcting the English grammar and language. The study was supported by grants NV18-07-00342 and RVO-VFN64165/2020 from the Czech Ministry of Health. The support of an intramural grant of Fondazione Italiana Fegato to Claudio Tiribelli is appreciated.
Author information
Authors and Affiliations
Contributions
Study concept and design, and retrieving and analyzing the literature: C.V.H., L.V., C.D.C.Z., C.C. and C.T. Drafting, and critical revision of the manuscript for important intellectual content: C.V.H., L.V., C.D.C.Z., A.D., P.S., E.A.E.H., C.C. and C.T. All authors approved the final manuscript as submitted
Corresponding author
Ethics declarations
Competing interests
C.T. is the President and C.D.C.Z. is the CTO of Bilimetrix, the company responsible for the development of the Bilistick® System. The remaining authors declare no competing interests.
Patient consent
Not required
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hulzebos, C.V., Vitek, L., Coda Zabetta, C.D. et al. Screening methods for neonatal hyperbilirubinemia: benefits, limitations, requirements, and novel developments. Pediatr Res 90, 272–276 (2021). https://doi.org/10.1038/s41390-021-01543-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01543-1
This article is cited by
-
Performance of smartphone application to accurately quantify hyperbilirubinemia in neonates: a systematic review with meta-analysis
European Journal of Pediatrics (2023)
-
5 Tage altes Neugeborenes mit „Gelbsucht“
Monatsschrift Kinderheilkunde (2022)