Abstract
Background
Preterm infants are at risk of neurodevelopmental impairments. At present, proton magnetic resonance spectroscopy (1H-MRS) is used to evaluate brain metabolites in asphyxiated term infants. The aim of this review is to assess associations between cerebral 1H-MRS and neurodevelopment after preterm birth.
Methods
PubMed and Embase were searched to identify studies using 1H-MRS and preterm birth. Eligible studies for this review included 1H-MRS of the brain, gestational age ≤32 weeks, and neurodevelopment assessed at a corrected age (CA) of at least 12 months up to the age of 18 years.
Results
Twenty papers evaluated 1H-MRS in preterm infants at an age between near-term and 18 years and neurodevelopment. 1H-MRS was performed in both white (WM) and gray matter (GM) in 12 of 20 studies. The main regions were frontal and parietal lobe for WM and basal ganglia for GM. N-acetylaspartate/choline (NAA/Cho) measured in WM and/or GM is the most common metabolite ratio associated with motor, language, and cognitive outcome at 18–24 months CA.
Conclusions
NAA/Cho in WM assessed at term-equivalent age was associated with motor, cognitive, and language outcome, and NAA/Cho in deep GM was associated with language outcome at 18–24 months CA.
Impact
-
In preterm born infants, brain metabolism assessed using 1H-MRS at term-equivalent age is associated with motor, cognitive, and language outcomes at 18–24 months.
-
1H-MRS at term-equivalent age in preterm born infants may be used as an early indication of brain development.
-
Specific findings relating to NAA were most predictive of outcome.
Similar content being viewed by others
Introduction
One in ten infants is born preterm. According to the World Health Organization, the annual number of preterm born infants is assumed to be ~15 million. Around one million children die every year because of prematurity-related morbidities.1 With advances in neonatal care, mortality after preterm birth has decreased. However, as a result of numerous risk factors preterm survivors, in particular those born before 32 completed weeks of gestation, are faced with a wide range of significant challenges of brain development.2 Long-term neurodevelopmental impairments (NDIs) like cerebral palsy (CP), neurocognitive, behavioral, and motor impairments affect nearly 25–35% of the preterm infants and increase with a decrease in gestational age (GA).3,4 Preterm infants have ~12-point lower intelligence quotient (IQ) levels,5 reduced language and motor abilities,6,7 attention difficulties and impaired social skills,8 and academic underachievement9 later in life. CP in preterm born infants may result from parenchymal brain injury such as periventricular hemorrhagic infarction or cystic periventricular leukomalacia, and can be predicted using term-equivalent age (TEA) magnetic resonance imaging (MRI). However, neurocognitive or behavioral impairments are commonly seen in extremely born preterm infants, and difficult to predict. Prediction of NDIs as a result of prematurity is crucial for proper clinical assessment and parental counseling, and guiding neurodevelopmental follow-up, as well as for the development of future neuroregenerative strategies.
MRI is increasingly used as a diagnostic tool for central nervous system evaluation since the early 1980s in both the neonatal and childhood period.10,11 Proton magnetic resonance spectroscopy (1H-MRS), a much older technique than MRI, has mainly been used for the prediction of outcome after perinatal asphyxia in term infants.12
During brain development, concentrations of metabolites change, with the most rapid alterations occurring within the first 2 years of life.13,14,15,16 It has been suggested that 1H-MRS metabolites are biomarkers for long-term neurodevelopment in preterm infants.
Therefore, the aim of the present review is to assess the association between cerebral 1H-MRS metabolites and neurodevelopmental outcome after preterm birth.
Methods
Design
A systematic search strategy was performed following the principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement in order to identify eligible studies.17 In this review, the aim was to identify and discuss all published 1H-MRS studies that predicted any neurodevelopmental outcome in infants (12–24 months), young children (2–6 years), and older children (6–18 years) born preterm ≤32 weeks.
Search strategy and information sources
Identification of studies was performed by an extensive search of electronic database Medline (PubMed, from 1995 to present, and Embase from 1988 to present). The last update of this search was on 31 May 2020. Entry terms were formulated based on the aim of the review, including premature infants, MRI techniques, and neurodevelopmental outcome, and were searched with the Medical Subject Heading search terms. Appendix 1 includes an overview of the entry terms and search strategy. The reference lists and bibliographies of the selected studies for inclusion were also manually reviewed to identify any other additional studies that were not included. The language choice for the published articles was limited to English and papers written in any other language were excluded. All prospective or retrospective human studies were included and no restriction was performed for the type of study to capture all eligible articles. Since the possibility of change in data after published version of studies, conference abstracts were not included in the review. Inclusion criteria were: (1) brain 1H-MRS acquired during the neonatal period and/or later ages, (2) prematurity at or below 32 weeks GA or very-low-birth-weight infants (birth weight <1500 g); (3) neurodevelopmental outcome assessment: CP, cognitive or intellectual impairments, social–emotional problems, and/or behavioral abnormalities diagnosed at a minimum age of 12 months corrected age (CA) up to 18 years.
Study selection
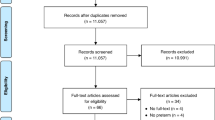
Title and abstract of studies were evaluated first, and if the screened articles met the inclusion criteria, then the full text of eligible articles was assessed. Two independent reviewers (B.C. and F.G.) read the full text of the selected studies. Records of screening and study selection are presented in Fig. 1. When debates occurred regarding the inclusion of studies, it was discussed with a third researcher (A.v.d.H.).
Data extraction
Data were extracted by two independent researchers (B.C. and F.G.) and crosschecked by a third researcher (T.A.). Detailed features of the articles (study design, sample size, 1H-MRS screening protocols, predictor metabolites, and neurodevelopmental tests and outcomes) were extracted to a specifically designed Excel workbook to classify the studies for the systematic review. When a 1H-MRS examination and neurodevelopmental follow-up were performed more than once, they were listed separately in the table (Table 3). When data were missing, the researchers contacted the author to request the data.
Methodological quality and synthesis
Included studies were critically appraised using the McMaster critical review form for quantitative studies18 and were comprehensively reviewed in terms of methodological quality. The review form consisted of 15 items including the risk of bias, and cut-off points were set according to the articles from the literature using this critical appraisal tool. Each point was assessed as fulfilled, partially fulfilled, and unfulfilled. Only items scored as fulfilled generated one point. A score of 13–15 was considered high quality, a score of 9–12 was moderate quality, and a score of ≤8 was low quality. If the included studies were heterogeneous, a narrative best-evidence synthesis was applied.
Results
Of 96 reported studies between 1995 and May 2020, 45 articles with results of preterm born infants underwent MRS screening. Among those 45 articles, 17 articles contained both 1H-MRS results and neurodevelopmental outcome and therefore met the inclusion criteria. Three additional articles were found after reviewing references of the initially included articles. Overall, 20 published articles examined brain metabolites using 1H-MRS and performed neurodevelopmental assessments in preterm infants and ten of these studies (four high-quality and six moderate-quality studies) reported significant associations between 1H-MRS metabolites and neurodevelopmental outcome (see PRISMA flowchart in Fig. 1). All characteristics of the studies (design, quality, number and GA of study population, age at MRI, age at follow-up, neurodevelopmental test, and association between 1H-MRS metabolites and neurodevelopmental outcome) are summarized in Table 1. All included studies showed a total score of 10–13 points according to the McMaster critical review form for quantitative studies. Five of these studies were considered as high quality and 15 were moderate quality. After summarizing the included studies, it became apparent that the included studies were heterogeneous, having a diversity of study settings and approaches. To synthesize the methodological quality of the studies and to enable conclusions to be drawn, a narrative best-evidence synthesis was applied.
Overarching outcome and subheadings from the narrative synthesis are described as follows:
-
1.
Magnetic resonance spectroscopic imaging (field strength, echo time (TE), and voxel dimensions).
-
2.
Voxel localization (white matter (WM), gray matter (GM), and cerebellum).
-
3.
1H-MRS metabolites (N-acetylaspartate (NAA), creatine (Cr), choline (Cho), myo-inositol (Ins), lactate (lac), and glutamate/glutamine (Glx)).
-
4.
Neurodevelopmental outcome.
Magnetic resonance spectroscopic imaging
Field strength
Eight recent studies used a 3.0-Tesla scanner,19,20,21,22,23,24,25,26 the other studies used 1.5 T equipment. 1H-MRS screening protocol and results of included studies are given in Table 2.
Echo time
Among the studies included in the systematic review, Simões et al.24 used a short TE (30 ms), whereas a long TE (272 ms) was reported in the study of Groenendaal et al.27 Both short and long TEs were used in two studies.15,26 Lac was utilized at TE ranging from 135 to 272 ms in four of the studies27,28,29,30 and short TE (of 35 ms) was used in one study to assess Lac.31
Voxel dimensions
Single voxel spectroscopy uses one volume of interest. It is the most commonly used acquisition method that can be easily implemented and processed. Two-dimensional (2D) MRS imaging is an extension of the single voxel to a slice, in which the voxels are phase-encoded. The 2D can be further extended to 3D to simultaneous coverage in the z/Feet-Head/caudal–cranial direction. The more extensive coverage of 2D and 3D acquisitions provides more informations, but requires more scanning time. 1H-MRS screening applied single voxel spectroscopy in 17 studies and only Hyodo et al.22 used 2D and Chau et al.32 and Xu et al.33 used 3D spectroscopy.
Voxel localization
White matter
Four studies selected a region of interest in the WM for the prediction of NDI.19,24,28,29 Of these studies, two were of high quality19,24 and two were of moderate quality;28,29 all showed a significant association with neurodevelopmental outcome (motor, cognitive, disabilities, IQ, and memory function). One of the four studies showed an association between low total Cho (tCho)/total creatine (tCr) ratio in preterm born infants and long-term adverse IQ, memory, and attention performance.19 Two of the studies specifically studied metabolites in the periventricular WM, which is known to be the most vulnerable area as it includes a high proportion of oligodendrocytes.28,29 In most of the studies, 1H-MRS was performed in both WM and GM with the frontal lobe being the most common region to place the VOI.20,21,22,23,31,33,34 Three of the included studies demonstrated an association between 1H-MRS and developmental outcomes both at 18 months CA and at 3–4 years of age (cognition, language, and motor disabilities).21,22,31
Gray matter
Three of the studies with moderate quality performed 1H-MRS only in the GM.25,35,36 Hippocampus, basal ganglia, and thalamus were the areas most frequently studied. No association between 1H-MRS metabolites and neurodevelopmental outcome was reported in these studies. The VOI was most often placed in the basal ganglia and motor deficit was the prominent NDI in these studies.22,27,31,32
The combination of 1H-MRS in both GM and WM was reported in 12 of 20 studies.20,21,22,23,26,27,31,32,33,36,37,38 Adverse neurodevelopmental outcomes related to 1H-MRS metabolites were reported in five studies, one study of high quality21 and four studies of moderate quality.22,27,31,32 Among these five studies, two studies21,27 reported a significant association between metabolites only in WM areas and adverse motor and cognitive outcome, whereas two studies22,31 found significant associations with motor impairment in only GM located metabolites. One study32 reported the association between metabolites in both WM and GM voxels and NDI (adverse cognitive outcome in WM and GM, and adverse motor outcome in WM).
Cerebellum
Only one study of high quality evaluated the cerebellum for 1H-MRS and reported a significant association between cerebellar metabolites and low cognitive scores at 2 years of age.26
1H-MRS metabolites
Main metabolites and factors affecting the alterations in concentrations will be discussed in detail for each metabolite.
N-acetylaspartate
NAA, a metabolite present in neurons, is synthesized in the mitochondria and decreases after injury as a result of neuronal integrity loss, but is also present in immature oligodendrocytes.39,40
All of the 20 studies evaluated NAA as a metabolite, given as concentration or as the ratio of various metabolites. NAA/Cho ratio was the most commonly used metabolite ratio in WM alone or together with GM areas predicting motor, language, and cognitive outcome,21,22,26,27,28,32 and NAA/Cr was the second most commonly used metabolite ratio and was predictive of motor, cognitive, and language scores as well as memory and attention as a long-term outcome measure in the study by Cheong and co-workers.19,20,24,28,31 Only one study of high quality demonstrated the predictive value of NAA/Ins ratio with the Bayley Scales of Infant Development Third Edition (BSID-III) at 18–22 months CA.21 NAA concentrations were utilized in two studies (one study of high quality23 and one study of moderate quality20), but no association was found with neurodevelopmental assessment.
The relationship between metabolite ratios and neurodevelopmental outcome at different age of MRS scan is given in Table 3.
Creatine
Cr is the primary supply for cellular energy metabolism. It is synthesized in the kidneys and liver and is then carried to the brain to maintain adenosine triphosphate in neurons.41 Cr peak consists of free Cr and phosphocreatine.40 Cr is generally utilized as a reference metabolite in ratios since its levels remain constant after the first year of life.42 Fourteen of 20 included studies used Cr in peak ratios with several metabolites. As a predictor for the neurodevelopmental outcome, NAA/Cr was the most common metabolite ratio in the studies in both WM and GM regions at 18–22 months CA as well as at 4 and 18 years of age.19,22,24,28,31 One study of high quality and two studies of moderate quality reported an association between Cho/Cr metabolite ratio and motor, expressive language, and memory scores.19,29,31 Cr concentrations were reported in two studies without any evident relation with outcome.20,23
Choline
Cho or tCho is used to address the signals of Cho-containing compounds such as free Cho, phosphocholine (PC), and glycerophosphocholine (GPC), which resonate at almost the same frequency. PC and GPC are found in phospholipids (phosphatidyl Cho and sphingomyelin), which are the main components of cell membranes.43 Cho is therefore accepted as an indication of structural integrity. Cho is also a precursor of acetylcholine, which is the main neurotransmitter in the brainstem responsible for signaling pathways.44 Cho levels increased in case of cellular proliferation, membrane turnover, myelination, or inflammation45,46,47,48,49 and decreased with age as the rapid brain growth in the neonatal period decelerates later in childhood.33
Overall, 20 studies involved the Cho peak or concentration to evaluate the adverse developmental outcome. Eight of the studies studied Cho in ratios of either NAA or Cr and reported an association with NDI.19,21,22,26,27,29,31,32 NAA/Cho ratio in frontal WM and deep GM nuclei was the most common predictor of NDI in one study of high quality21 and four studies of moderate quality22,27,31,32 and Cho/Cr ratio was the metabolite ratio to predict adverse developmental outcome including both motor and cognitive problems at childhood and adolescence period.19,31 Low Cho/Cr level was significantly associated with motor impairments in childhood period31 and higher tCho/tCr in GM was significantly correlated with higher IQ in preterm born adolescents.19
Myo-inositol
Ins is the precursor metabolite of phosphatidylinositol, which is essential for signal transduction, especially in WM.50 It has a crucial role in the regulation of extracellular osmolality51,52 and is accepted as a marker of gliosis in the brain.53 It increases in the early stage of hypoxia–ischemia and has a decreasing trend in the perinatal period.54 Ins was evaluated in 9 of 20 included studies20,21,23,24,25,26,31,36,37 and an association between metabolites and NDI was noted in two studies of high quality21,24 and one study of moderate quality.31 Among these studies, one study of high quality reported NAA/Ins ratio in the WM was significantly associated with BSID-III mental and cognitive scores at 18–22 months CA.21
Lactate
Lac is accepted as hypoxic and/or ischemic marker. At a TE of 144 ms, Lac is fully inverted and a TE of 288 ms allows Lac to be seen in a completely upright position and reduces the amount of lipid contamination.55 Elevated Lac is indicative of brain parenchyma ischemia after underlying nonoxidative glucose consumption. Several studies of term neonates with encephalopathy demonstrated that an increase in Lac concentration and a decrease in NAA concentration is correlated with NDI.56,57,58,59,60,61,62 A meta-analysis about neonatal encephalopathy in (near) term infants reported that Lac/NAA ratio in deep GM is the most precise biomarker currently available for prediction of adverse neurodevelopmental outcome.63 Elevation of Lac in preterm born infants might be interpreted as normal because of usual alterations in metabolism in the preterm brain.2,64,65,66 Lac was evaluated in 8 of 20 included studies,15,26,27,28,29,30,31,33 and among these eight studies, five studies reported an association between metabolites and NDI, and only study of Hart et al.28 with moderate quality identified high Lac doublet to be significantly associated with fine motor scores of BSTID-III.
Glutamate/glutamine
Glutamate, the major component of the Glx peak including γ-aminobutyric acid, glutamine, and glutamate, is the most abundant excitatory neurotransmitter in all brain regions. Increased glutamate concentration after hypoxia causes toxicity to neurons, which ends up in cell injury and/or death.67 Five of the included studies used Glx either in metabolite ratios or as Glx concentrations.15,20,23,24,25 No association was reported between Glx and neurodevelopmental outcome in two studies of high quality and three studies of moderate quality.
Neurodevelopmental outcome
The neurodevelopmental assessment was conducted with the BSID-III in ten of the included studies,21,23,24,26,28,29,30,32,33,38 and among these studies, three studies of high quality21,24,26 and three studies with moderate quality28,29,32 demonstrated adverse neurodevelopmental outcome relative to neurometabolites. Two studies estimated NDI using other tests. The Wechsler Preschool and Primary Scale of Intelligence-III test was used as a developmental test tool at 3–4 years of age in the study of Phillips et al.23 and Cheong et al.19 performed a two-subtest version of the Wechsler Abbreviated Scale of Intelligence test at 18 years. Motor disability was the main reported outcome in six studies22,24,27,29,31,32 and it was mostly associated with NAA/Cho ratios in WM at 18–24 months CA in one study with high quality24 and two studies with moderate quality.22,27 Only one study with moderate evidence assessed developmental delay at 3.5–4.5 years reporting lower Cho/Cr ratios in both WM and GM.31 NAA/Cho ratios in WM were associated with low cognitive scores at 18–24 months CA in three of the studies.24,26,32 Low NAA/Cho ratios in both WM and GM were significantly associated with lower language score in two studies in preterm born infants assessed at 18–24 months CA in two studies of high quality.21,24 Only Cheong et al.19 reported that low Cho/Cr ratios correlated with lower IQ in preterm born adolescents.
Discussion
This systematic review included 20 studies investigating the predictive role of 1H-MRS metabolites in neurodevelopmental outcome in infants (12–24 months), young children (2–6 years), and older children (6–18 years) in infants born preterm. Ten of the studies reported the association between 1H-MRS metabolites and NDI.
Conventional MRI is not able to define changes in cellular biochemical composition and structure that can be available with 1H-MRS. The number and quantitation of detectable metabolites depend on the pulse sequence and its parameters, besides spectral resolution and signal-to-noise ratio (SNR).68,69 Metabolites obtained from 1H-MRS are reported most frequently as either peak–area ratios or absolute concentrations. Measurements of absolute concentrations of metabolites have been performed by using external or internal standards, although both methods have some disadvantages. Using external standards is inconvenient and time-consuming,70,71 and use of water as the internal standard has the disadvantage of the assumption that brain water content is constant, which is not true in different brain diseases such as post hypoxia–ischemia. During brain development, concentrations of several neurochemicals change, resulting in various alterations that occur during the first 2 years of life.15,16,72 The maturational pattern of preterm infants is different compared to term infants. In preterm infants, NAA, Cr, glutamate increase, and Ins and Lac decrease towards TEA.73
Most of the tissue abnormalities seen by MRI in preterm infants are observed in the WM, including small punctate or cystic lesions, diffuse excessive high signal intensity, impaired myelin maturation, parenchymal tissue loss, and corpus callosum abnormalities.74,75,76 Disrupted maturation and chronic myelination disturbances are accepted as the main underlying pathologies77 and CP is the most common cause of long-term NDI in children with severe WM injury.78,79,80 Since the largest number of developing oligodendrocytes are localized in the posterior periventricular white matter, this area is one of the most vulnerable regions in the preterm brain.81
GM is composed of the cerebral cortex and deep central nuclei (basal ganglia, thalamus), which provide interconnection between cerebral cortex and several other brain areas. Preterm infants with GM damage are at risk for long-term neurocognitive impairments via direct or secondary injury to sensory and motor axons.2 Some studies revealed that reduced cortical and deep GM volume in preterm born infants had an association with moderate-to-severe NDI at 12 months CA compared to term infants.37,82 Additionally, cognitive disabilities including working memory and IQ were found to be correlated with GM damage.83,84,85,86,87 Any WM injury is also assumed to disturb the development of GM affecting motor, cognitive, and intellectual outcomes.88,89,90,91 The cerebellum provides interconnection with the cerebral hemispheres and processes in higher functioning, such as motor functions, as well as learning, memory, cognitive, and behavioral functions.92,93 Cerebellar hemorrhage is a serious and not well-recognized complication in preterm born infants related with high mortality and NDI.94,95,96,97
Our review demonstrates that in preterm infants, 1H-MRS performed in WM areas at TEA is associated with neurodevelopmental outcome at 18–24 months CA. Motor disability at 12–24 months CA is the most commonly reported adverse finding among all other evaluated parameters, and low cognitive score was the second common outcome performed at 18–24 months CA. Most studies evaluated 1H-MRS at TEA and a few studies did a scan at a later age including four studies at 4–6 years of age,20,23,31,36 one study at 8 years of age,35 and 1 study at 18 years of age.19 Several studies had repeated MRI scans at different time points20,22,23,25,26,30,32,36 and two studies (one study of high and one study of moderate quality) performed more than one neurodevelopmental assessment at different time period.20,23 Only one study of moderate quality found a relation between an early 1H-MRS age (30–34 weeks) with an adverse motor and cognitive outcome.32 The region with the highest association between brain metabolites and neurodevelopmental outcome cannot be identified based on data from the present review. More studies measuring various brain areas are necessary to clarify this. There are limited data assessing other cognitive, behavioral, and language scores at a later age so that further studies are needed to evaluate long-term neurodevelopmental outcome.
Studies included in this systematic review were composed of variable populations involving both a small number (range 12–43) and a large number of infants (range 65–177) born at or below 32 gestational weeks. The number of infants that participated in the majority of the studies was <30. More than half of the studies had a control group consisting of term babies to compare with the preterm infants. Only one study of moderate quality reported sensitivity and specificity to define predictive value of metabolites for neurodevelopmental outcome.29 In addition, only two studies reported the relation between IUGR in preterm infants and 1H-MRS metabolic ratios.15,24 Therefore, no definite conclusions on the effects of IUGR in preterm infants can be made.
Detection of in vivo biochemical data provides not only a perspective to the metabolic assessment that enlighten brain development, but also may enable a better insight to alterations of metabolites in case of abnormal processes.13 Several metabolites were measured to predict neurodevelopmental outcome, however, mostly restricted to the first 2 years of life with limited data for later ages. NAA/Cho ratio has the highest predictive value for motor impairments in infants, but still there is a lack of data to predict cognitive impairments because outcome reports of school-aged children were limited. The use of NAA as a biomarker for neurodevelopment carries a high potential given the almost exclusive presence of NAA in neurons and immature oligodendrocytes, cells that have a particular vulnerability in the preterm brain.
Concerning field strength, the older studies have been performed in using a 1.5 T system, the newer studies used 3.0 T equipment, which may improve the identification of metabolites. Studies performed with 3.0 T report tissue levels for metabolites such as Ins, Glx, and gamma-aminobutyric acid (GABA) as quantification of these metabolites is more reliable at 3.0 T because of its increased SNR. Recently, the first study of human infants in a 7 T field was published, which demonstrated increased chemical shift dispersion and less overlap between the different metabolite peaks, which benefits the detection of the overlapping signals of, e.g., glutamate, glutamine, and GABA.98 For the major signals in (ischemic) brain, such as NAA, Cho, and Lac, the use of 1.5 T or 3.0 T 1H-MRS did not result in different clinical decision-making.99
TE is important in the identification of metabolites. 1H-MRS at short TE enables the quantification of a comprehensive biochemical profile including several brain metabolites.100 More compounds can be detected using shorter TEs (25–35 ms) compared to longer TEs (144 or 288 ms). The disadvantage of the short TE might be the presence of broad signal from high-molecular-weight macromolecules and lipids,101 which have a relatively short T2. Longer TEs are mainly preferred to discriminate Lac from lipids and overlapping multiplets.55,102
There are several limitations of the data used in our review. 1H-MRS techniques varied among the studies including different TEs, magnetic field strengths, preferred regions, and voxel size, which may affect tissue specificity and resulted in regions of interest that include nontargeted tissues. The larger chemical shift dispersion at higher field strengths provides a better discrimination of individual components, and SNR is improved. Ideally, raw data need to be processed using the same processing pipeline to compare 1H-MRS results between different scanners. Recently, recommendations on single voxel MRS data processing have been summarized by the MRS experts’ working group.103 The methods used in the main steps, preprocessing, analysis, and quantification, should be reported along with study results to be able to compare results from different studies. The reporting of data processing methods for most studies in the present review did not meet the new standards as proposed by the MRS expert’s working group.104 Nevertheless, by comparing ratios of metabolite levels and not the absolute concentrations of metabolites, the effect of differences in processing methods on clinically relevant parameters is largely reduced.
There were insufficient data investigating metabolites like glutamate, taurine, lipids, and other macromolecules, which might be associated with neurodevelopment. Neurodevelopmental assessment in the included studies was evaluated with a variety of developmental tests and the results may be affected by several variables as linguistic, socio-economic, and cultural patterns.
Future developments
1H-MRS plays a special role in the assessment of brain development, providing information on the molecular composition of brain tissue. Use of chemical shift imaging preferably at higher magnetic field strength to reduce scanning time will enable clinicians to obtain detailed information on metabolites of several brain structures, including periventricular WM, deep GM, and the cerebellum, which are all known to be at risk in preterm neonates.
With short scanning times, metabolic imaging can be added to the standard imaging protocol even in very preterm and vulnerable patients. Multicenter studies in preterm infants will be needed to compare findings obtained in different scanners, which will facilitate the use of 1H-MRS as a surrogate end point in clinical trials.
Conclusion
1H-MRS is a potential surrogate end point for neurodevelopment in preterm infants: NAA/Cho ratio in WM at term equivalent age is associated with motor outcome in preterm infants at 18–24 months’ CA. NAA/Cho ratios in the WM were associated with cognitive scores and NAA/Cho ratios in the WM and GM were significantly related to language scores in preterm born infants assessed at 18–24 months’ corrected age. There is a need for further studies evaluating the association between neonatal 1H-MRS in preterm infants and long-term neurodevelopmental outcome.
References
Liu, L. et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 388, 3027–3035 (2016).
Panigrahy, A. et al. Neuroimaging biomarkers of preterm brain injury: toward developing the preterm connectome. Pediatr. Radiol. 42, 33–61 (2012).
Aarnoudse-Moens, C. S. et al. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 124, 717–728 (2009).
Saigal, S. & Doyle, L. W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371, 261–269 (2008).
Kerr-Wilson, C. O., Mackay, D. F., Smith, G. C. S. & Pell, J. P. Meta-analysis of the association between preterm delivery and intelligence. J. Public Health 34, 209–216 (2011).
de Kieviet, J. F. et al. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev. Med. Child Neurol. 54, 313–323 (2012).
van Noort-van der Spek, I. L., Franken, M. C. J. P. & Weisglas-Kuperus, N. Language functions in preterm-born children: a systematic review and meta-analysis. Pediatrics 129, 745–754 (2012).
Johnson, S. et al. Neurodevelopmental disability through 11 years of age in children born before 26 weeks of gestation. Pediatrics 124, e249–e257 (2009).
Moster, D., Lie, R. T. & Markestad, T. Long-term medical and social consequences of preterm birth. N. Engl. J. Med. 359, 262–273 (2008).
Smith, F. W. The value of NMR imaging in pediatric practice—a preliminary report. Pediatr. Radiol. 13, 141–147 (1983).
Johnson, M. A. et al. Clinical NMR imaging of the brain in children—normal and neurologic disease. Am. J. Neuroradiol. 4, 1013–1026 (1983).
Groenendaal, F. & de Vries, L. S. Fifty years of brain imaging in neonatal encephalopathy following perinatal asphyxia. Pediatr. Res. 81, 150–155 (2017).
Moore, G. J. Proton magnetic resonance spectroscopy in pediatric neuroradiology. Pediatr. Radiol. 28, 805–814 (1998).
Kreis, R. et al. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn. Reson. Med. 48, 949–958 (2002).
Roelants-van Rijn, A. M., van der Grond, J., Stigter, R. H., de Vries, L. S. & Groenendaal, F. Cerebral structure and metabolism and long-term outcome in small-for-gestational-age preterm neonates. Pediatr. Res. 56, 285–290 (2004).
Heerschap, A., Kok, R. D. & van den Berg, P. P. Antenatal proton MR spectroscopy of the human brain in vivo. Childs Nerv. Syst. 19, 418–421 (2003).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8, 336–341 (2010).
Law, M. et al. Guidelines for Critical Review of the Literature: Quantitative Studies, Vol. 14, 1–11 (McMaster University, 1998).
Cheong, J. L. et al. Altered posterior cingulate brain metabolites and cognitive dysfunction in preterm adolescents. Pediatr. Res. 79, 716–722 (2016).
Gasparovic, C. et al. The long-term effect of erythropoiesis stimulating agents given to preterm infants: a proton magnetic resonance spectroscopy study on neurometabolites in early childhood. Pediatr. Radiol. 48, 374–382 (2018).
Bapat, R., Narayana, P., Zhou, Y. & Parikh, N. Magnetic Resonance spectroscopy at term equivalent age in extremely preterm infants: association with cognitive and language development. Pediatr. Neurol. 51, 53–59 (2014).
Hyodo, R. et al. Magnetic resonance spectroscopy in preterm infants: association with neurodevelopmental outcomes. Arch. Dis. Child Fetal Neonatal Ed. 103, 238–244 (2018).
Phillips, J. P. et al. Anterior cingulate and frontal lobe white matter spectroscopy in early childhood of former very LBW premature infants. Pediatr. Res. 69, 224–229 (2011).
Simões, R. V. et al. Brain metabolite alterations in infants born preterm with intrauterine growth restriction: association with structural changes and neurodevelopmental outcome. Am. J. Obstet. Gynecol. 216, 1–14 (2017).
Tanifuji, S. et al. Temporal brain metabolite changes in preterm infants with normal development. Brain Dev. 39, 196–202 (2017).
Van Kooij, B. J. et al. Cerebellar volume and proton magnetic resonance spectroscopy at term, and neurodevelopment at 2 years of age in preterm infants. Dev. Med. Child Neurol. 54, 260–266 (2012).
Groenendaal, F. et al. Early cerebral proton MRS and neurodevelopmental outcome in infants with cystic leukomalacia. Dev. Med. Child Neurol. 39, 373–379 (1997).
Hart, A. R. et al. Diffusion-weighted imaging and magnetic resonance proton spectroscopy following preterm birth. Clin. Radiol. 69, 870–879 (2014).
Kendall, G. S. et al. White matter NAA/Cho and Cho/Cr ratios at MR spectroscopy are predictive of motor outcome in preterm infants. Radiology 271, 230–238 (2014).
Podrebarac, S. K. et al. Antenatal exposure to antidepressants is associated with altered brain development in very preterm-born neonates. Neuroscience 7, 252–262 (2017).
Durlak, W. et al. Relationship between proton magnetic resonance spectroscopy of frontoinsular gray matter and neurodevelopmental outcomes in very low birth weight children at the age of 4. PLoS ONE 11, e0156064 (2016).
Chau, V. et al. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology 81, 2082–2089 (2013).
Xu, D. et al. MR spectroscopy of normative premature newborns. J. Magn. Reson. Imaging 33, 306–311 (2011).
Akasaka, M. et al. Assessing temporal brain metabolite changes in preterm infants using multivoxel magnetic resonance spectroscopy. Magn. Reson. Med. Sci. 15, 187–192 (2016).
Rademaker, K. J. et al. Neonatal hydrocortisone treatment related to 1H-MRS of the hippocampus and short-term memory at school age in preterm born children. Pediatr. Res. 59, 309–313 (2006).
Taylor, M. J. et al. Magnetic resonance spectroscopy in very preterm-born children at 4 years of age: developmental course from birth and outcomes. Neuroradiology 60, 1063–1073 (2018).
Inder, T. E. et al. Abnormal cerebral structure is present at term in premature infants. Pediatrics 115, 286–294 (2005).
Augustine, E. M. et al. Can magnetic resonance spectroscopy predict neurodevelopmental outcome in very low birth weight preterm infants? J. Perinatol. 28, 611–618 (2008).
Bjartmar, C., Battistuta, J., Terada, N., Dupree, E. & Trapp, B. D. N-acetylaspartate is an axon-specific marker of mature White matter in vivo: a biochemical and immunohistochemical study on the rat optic nerve. Ann. Neurol. 51, 51–58 (2002).
Birken, D. L. & Oldendorf, W. H. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neurosci. Biobehav. Rev. 13, 23–31 (1989).
Braissant, O. et al. Creatine synthesis and transport during rat embryogenesis: spatiotemporal expression of AGAT, GAMT and CT1. BMC Dev. Biol. 5, 9 (2005).
Pouwels, P. J. et al. Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatr. Res. 46, 474–485 (1999).
Zeisel, S. H., Char, D. & Sheard, N. F. Choline, phosphatidylcholine and sphingomyelin in human and bovine milk and infant formulas. J. Nutr. 116, 50–58 (1986).
Brandon, E. P. et al. Choline transporter 1 maintains cholinergic function in choline acetyltransferase haplo insufficiency. J. Neurosci. 24, 5459–5466 (2004).
Stork, C. & Renshaw, P. F. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol. Psychiatry 10, 900–919 (2005).
Howe, F. A. et al. Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn. Reson. Med. 49, 223–232 (2003).
Miller, B. L. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate,creatine and choline. NMR Biomed. 4, 47–52 (1991).
Licata, S. C. & Renshaw, P. F. Neurochemistry of drug action: insights from proton magnetic resonance spectroscopic imaging and their relevance to addiction. Ann. NY Acad. Sci. 1187, 148–171 (2010).
Richards, T. L. Proton MR spectroscopy in multiple sclerosis: value in establishing diagnosis, monitoring progression, and evaluating therapy. Am. J. Roentgenol. 157, 1073–1078 (1991).
Berry, G. T. Is prenatal myo-inositol deficiency a mechanism of CNS injury in galactosemia? J. Inherit. Metab. Dis. 34, 345–355 (2011).
Lien, Y. H., Shapiro, J. I. & Chan, L. Effects of hypernatremia on organic brain osmoles. J. Clin. Invest. 85, 1427–1435 (1990).
Thurston, J. H., Sherman, W. R., Hauhart, R. E. & Kloepper, R. F. Myo-inositol: a newly identified nonnitrogenous osmoregulatory molecule in mammalian brain. Pediatr. Res. 26, 482–485 (1989).
Isaacks, R. E., Bender, A. S., Kim, C. Y., Prieto, N. M. & Norenberg, M. D. Osmotic regulation of myo-inositol uptake in primary astrocyte cultures. Neurochem. Res. 19, 331–338 (1994).
Robertson, N. J. et al. Early increases in brain myo-inositol measured by proton magnetic resonance spectroscopy in term infants with neonatal encephalopathy. Pediatr. Res. 50, 692–700 (2001).
Xu, D. & Vigneron, D. Magnetic resonance spectroscopy imaging of the newborn brain-a technical review. Semin. Perinatol. 34, 20–27 (2010).
Barkovich, A. J. et al. Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. Am. J. Neuroradiol. 20, 1399–1405 (1999).
Cheong, J. L. et al. Proton MR spectroscopy in neonates with perinatal cerebral hypoxic-ischemic injury: metabolite peak-area ratios, relaxation times, and absolute concentrations. Am. J. Neuroradiol. 27, 1546–1554 (2006).
Barkovich, A. J. et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. Am. J. Neuroradiol. 27, 533–547 (2006).
Miller, S. P. et al. Predictors of 30-month outcome after perinatal depression: role of proton MRS and socioeconomic factors. Pediatr. Res. 52, 71–77 (2002).
Shu, S. K., Ashwal, S., Holshouser, B. A., Nystrom, G. & Hinshaw, D. B. Prognostic value of 1H-MRS in perinatal CNS insults. Pediatr. Neurol. 17, 309–318 (1997).
Groenendaal, F. et al. Cerebral lactate and N-acetyl-aspartate/choline ratios in asphyxiated fullterm neonates demonstrated in vivo using proton magnetic resonance spectroscopy. Pediatr. Res. 35, 148–151 (1994).
Robertson, N. J. et al. Cerebral intracellular lactic alkalosis persisting months after neonatal encephalopathy measured by magnetic resonance spectroscopy. Pediatr. Res. 46, 287–296 (1999).
Thayyil, S. et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics 125, 382–395 (2010).
Coyle, J. T. The glutamatergic dysfunction hypothesis for schizophrenia. Harv. Rev. Psychiatry 3, 241–253 (1996).
Ueda, Y. et al. Collapse of extracellular glutamate regulation during epileptogenesis: downregulation and functional failure of glutamate transporter function in rats with chronic seizures induced by kainic acid. J. Neurochem. 76, 892–900 (2001).
Nguyen, L. et al. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 305, 187–202 (2001).
Manev, H., Favaron, M., Guidotti, A. & Costa, E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol. Pharmacol. 36, 106–112 (1989).
Fein, G. & Meyerhoff, D. J. Ethanol in human brain by magnetic resonance spectroscopy: correlation with blood and breath levels, relaxation, and magnetization transfer. Clin. Exp. Res. 24, 1227–1235 (2000).
Gruetter, R. et al. Resolution improvements in in vivo 1H NMR spectra with increased magnetic field strength. J. Magn. Reson. 135, 260–264 (1998).
Cheong, J. L. Y. et al. Proton MR spectroscopy in neonates with perinatal cerebral hypoxic-ischemic injury:metabolite peak-area ratios, relaxation times, and absolute concentrations. Am. J. Neuroradiol. 27, 1546–1554 (2006).
Provencher, S. W. Automatic quantitation of localized in vivoH 1 spectra with LCModel. NMR Biomed. 14, 260–264 (2001).
Moore, G. J. Proton magnetic resonance spectroscopy in pediatric neuroradiology. Pediatr. Radiol. 28, 805–814 (1998).
Anderson, P. J., Cheong, J. L. & Thompson, D. K. The predictive validity of neonatal MRI for neurodevelopmental outcome in very preterm children. Semin. Perinatol. 39, 147–158 (2015).
Smyser, C. D., Kidokoro, H. & Inder, T. E. Magnetic resonance imaging of the brain at term equivalent age in extremely premature neonates. J. Paediatr. Child Health 48, 794–800 (2012).
Inder, T. E. et al. Defining the nature of the cerebral abnormalities in the premature infant: aqualitative magnetic resonance imaging study. J. Pediatr. 143, 171–179 (2003).
Kidokoro, H., Neil, J. J. & Inder, T. E. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. Am. J. Neuroradiol. 34, 2208–2214 (2013).
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8, 110–124 (2009).
Bax, M., Tydeman, C. & Flodmark, O. Clinical and MRI correlates of cerebral palsy: the European Cerebral Palsy Study. JAMA 296, 1602–1608 (2006).
Himpens, E. et al. Prevalence, type, distribution, and severity of cerebral palsy in relation to gestational age: a meta- analytic review. Dev. Med. Child Neurol. 50, 334–340 (2008).
Spittle, A. J. et al. Neonatal white matter abnormality predicts childhood motor impairment in very preterm children. Dev. Med. Child Neurol. 53, 1000–1006 (2011).
Riddle, A. et al. Spatial heterogeneity in oligodendrocyte lineage maturation and not cerebral blood flow predicts fetal ovine periventricular white matter injury. J. Neurosci. 26, 3045–3055 (2006).
Peterson, B. S. et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics 111, 939–948 (2003).
Soria-Pastor, S. et al. Decreased regional brain volume and cognitive impairment in preterm children at low risk. Pediatrics 124, 1161–1170 (2009).
Peterson, B. S. et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA 284, 1939–1947 (2000).
Anderson, P. & Doyle, L. W. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA 289, 3264–3272 (2003).
Woodward, L. J. et al. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain 128, 2578–2587 (2005).
Beauchamp, M. H. et al. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain 131, 2986–2994 (2008).
Leviton, A. & Gressens, P. Neuronal damage accompanies perinatal white-matter damage. Trends Neurosci. 30, 473–478 (2007).
Carpenter, K. L. H. et al. Magnetic susceptibility of brain iron is associated with childhood spatial IQ. Neuroimage 132, 167–174 (2016).
Srinivasan, L. et al. Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images. Pediatrics 119, 759–765 (2007).
Pierson, C. R. et al. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 114, 619–631 (2007).
Baillieux, H., De Smet, H. J., Paquier, P. F., De Deyn, P. P. & Marien, P. Cerebellar neurocognition: insights into the bottom of the brain. Clin. Neurol. Neurosurg. 110, 763–773 (2008).
Tavano, A. et al. Disorders of cognitive and affective development in cerebellar malformations. Brain 130, 2646–2660 (2007).
Limperopoulos, C. et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 120, 584–593 (2007).
Limperopoulos, C. et al. Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors. Pediatrics 116, 717–724 (2005).
Bednarek, N. et al. Outcome of cerebellar injury in very low birth-weight infants: 6 case reports. J. Child Neurol. 23, 906–911 (2008).
Tam, E. W. et al. Cerebellar hemorrhage on magnetic resonance imaging in preterm newborns associated with abnormal neurologic outcome. J. Pediatr. 15, 245–250 (2011).
Annink, K. V. et al. Introduction of ultra-high-field MR imaging in infants: preparations and feasibility. Am. J. Neuroradiol. 41, 1532–1537 (2020).
Alderliesten, T. et al. MRI and spectroscopy in (near) term neonates with perinatal asphyxia and therapeutic hypothermia. Arch. Dis. Child Fetal Neonatal Ed. 102, F147–F152 (2017).
Tkáč, I., Öz, G., Adriany, G., Uǧurbil, K. & Gruetter, R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn. Reson. Med. 62, 868–879 (2009).
Cudalbu, C., Mlynárik, V. & Gruetter, R. Handling macromolecule signals in the quantification of the neurochemical profile. J. Alzheimer’s Dis. 31, 101–115 (2012).
Wilson, M. et al. Methodological consensus on clinical proton MRS of the brain: review and recommendations. Magn. Reson. Med. 82, 527–550 (2019).
Near, J. et al. Preprocessing, analysis and quantification in single-voxel magnetic resonance spectroscopy: experts’ consensus recommendations. NMR Biomed. https://doi.org/10.1002/nbm.4257 (2020).
Lin, A. et al. Minimum Reporting Standards for in vivo Magnetic Resonance Spectroscopy (MRSinMRS): experts’ consensus recommendations. NMR Biomed. https://doi.org/10.1002/nbm.4484 (2021).
Acknowledgements
A grant from the “Stichting Neonatale Neurologie Utrecht” (www.snnu.nl) was received to cover the publication costs.
Author information
Authors and Affiliations
Contributions
B.C., F.G., A.v.d.H., and T.A.: substantial contributions to conception and design; B.C., F.G., and A.v.d.H.: acquisition of data; B.C., T.A., J.P.W., A.v.d.H., F.G.: analysis and interpretation of data; B.C. and F.G.: drafting the article or revising it critically for important intellectual content; all authors: final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Cebeci, B., Alderliesten, T., Wijnen, J.P. et al. Brain proton magnetic resonance spectroscopy and neurodevelopment after preterm birth: a systematic review. Pediatr Res 91, 1322–1333 (2022). https://doi.org/10.1038/s41390-021-01539-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01539-x