Abstract
Background
Late and moderate prematurity may have an impact on pulmonary function during childhood. The present study aimed to investigate lung mechanics in school-age children born moderate-to-late preterm (MLPT).
Methods
Children aged 5–10 years were enrolled in this case–control study. Lung function and bronchodilator response were assessed by impulse oscillometry (IOS) at two hospital-based specialized clinics. A structured questionnaire was employed to assess respiratory morbidities.
Results
A total of 123 children was divided into two groups: case (MLPT) n = 52 and control (children born at term) n = 71. The results showed no difference between groups in mean baseline IOS variables: R5 0.80 ± 0.20 vs 0.82 ± 0.22 kPa/L/s, p = 0.594, R20 0.54 ± 0.13 vs 0.55 ± 0.13 kPa/L/s, p = 0.732, R5–R20 0.26 ± 0.12 vs 0.27 ± 0.15 kPa/L/s, p = 0.615, X5 −0.29 ± 0.01 vs −0.29 ± 0.1 kPa/L/s, p = 0.990, Fres 21.1 ± 3.3 vs 21.7 ± 3.1 L/s, p = 0.380, and AX 2.7 ± 3.36 vs 2.5 ± 1.31 kPa/L/s, p = 0.626. Bronchodilator response and the occurrence of respiratory morbidities after birth were also similar between groups.
Conclusions
This study found lung mechanics parameters to be similar in school-age children born MLPT and those born at term, suggesting that pulmonary plasticity continues to occur in children up to school age.
Impact
-
Late and moderate prematurity is associated with an increased risk of reduced pulmonary function during childhood.
-
Follow-up reports in adolescents and adults born MLPT are scarce but have indicated pulmonary plasticity with normalization of airway function.
-
Our results show that the lung function in school-age children born MLPT is similar to that of children born at term.
Similar content being viewed by others
Introduction
Advances in perinatal care practices have improved the prognosis regarding survival for premature newborns.1,2,3 Despite this, prematurity is still a serious and growing global health problem2 and a significant proportion of these infants are afflicted by dysfunctions that may exacerbate the occurrence of respiratory morbidities later in life. Coughing, wheezing, pneumonia, asthma, increased use of inhaled medications, and hospitalizations are common among such children when they reach school age.4,5,6,7,8,9,10
Morphological and functional changes that begin in the late prenatal period prepare the respiratory system for its essential postnatal roles and ensure its autonomy once the placenta is separated.3 Preterm birth can compromise lung maturation and predispose children to postnatal respiratory morbidities that can be aggravated by interventions such as mechanical ventilation and oxygen therapy.11
Pulmonary dysfunction and respiratory morbidities are not restricted to extremely or very preterm infants. There seems to be an agreement among studies that lung dysfunction is also relatively common in early childhood in those born moderate and late preterm.4,5,6,7,8,9 In clinical practice, however, such children are often considered physiologically and metabolically similar to those born at term.8
Nonetheless, it is not clear whether these changes persist over time or whether they can be influenced by pulmonary plasticity. There is a paucity of data reporting lung function in infancy and in children born moderate-to-late preterm. The present study aims to assess lung mechanics using IOS and, as a secondary outcome, to investigate the occurrence of respiratory morbidities in school-age children born moderate-to-late preterm.
Methods
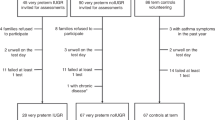
This bicentric case–control study was approved by the institutional review board (protocol number 1.063.394/CAAE 34622714.0.3001.5201). A convenience sample was put together of children presenting at a public hospital-based outpatients clinic in the Northeast region of Brazil.
The study included schoolchildren of both sexes, aged between 5 and 10 years, and born moderate-to-late preterm (32–<37 full weeks of gestation). The control group consisted of children with the same characteristics born at term. Children with a history of intrauterine growth restriction, bronchopulmonary dysplasia, and non-pulmonary conditions (heart conditions or rheumatologic, neuromuscular, or orthopedic disorders) capable of altering lung function or causing respiratory infection up to 2 weeks prior to the evaluation were excluded from the study.
The children’s caregivers/guardians were informed about the study and, upon agreeing to participate, signed the informed consent form and received information on how to suspend medication (when in use), according to the American Thoracic Society/European Respiratory Society Statement (ERS/ATS) recommendations.12,13 The children’s medical records were consulted to gather epidemiological and clinical data and to confirm the inclusion criteria. Perinatal and postnatal data and personal data for each child were also collected by way of an interview and consultation of hospital records.
Study protocol
Lung function was assessed using IOS by a single researcher, on an IOS Jaeger™ Master Screen™ system (Erich Jaeger, Germany) (IOS module) as per ERS standards.14 Children were instructed to spontaneously breathe at tidal volume while wearing a mouthpiece. The following parameters were taken into account: total (R5) and central (R20) respiratory resistance, frequency dependence of resistance (R5–R20), respiratory reactance (X5), resonant frequency (Fres), and reactance area (AX).
A maximum variance of 15% between measurements was tolerated for each of the parameters. Examinations not achieving the expected reliability were discarded. The average of the measurements derived from three technically acceptable maneuvers was used for each variable studied and compared to the average predicted for age, sex, weight, and height. Predicted values were obtained using the equations proposed by Dencker et al.15 and Malmberg et al.16
The means of R5 and X5 measurements were also analyzed according to the cut-off points considered for normality of variance in various studies,12,17 “<150% of the predicted value” and “< predicted value − 0.15,” respectively, and compared between groups.
After baseline measurements, salbutamol spray (100 µg) was administered to identify the variation of oscillometric measurements in response to the bronchodilator. The vial was agitated prior to use and three medication puffs (300 μg) were administered orally, using a spacer connected to the mouthpiece. The child was instructed to remain in a sitting position and adopt a slow and deep oral breathing pattern for at least 20 s. A nasal clip was used during the procedure.
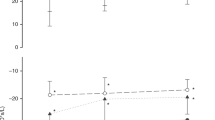
After 15 min of bronchodilator administration, IOS was repeated. Values were expressed as a percentage of the baseline value [(post-value − pre-value/pre-value) × 100], difference between the absolute values pre- and post-bronchodilator (Δabsolute) and percentage of the predicted value [(post-value − pre-value/value predicted) × 100]).18,19 The averages of the values found were compared between the groups and evaluated, with a positive bronchodilator response considered to be a reduction >40% in R5 and an increase >50% in X5.14
A structured questionnaire was also applied to assess respiratory morbidities. The questions were answered by the children’s caregivers/guardians. Each one of the pulmonary morbidities and the sum of positive responses for each question were compared between groups. Sex of participants was assigned following external examination of body characteristics.
Statistics
The Kolmogorov–Smirnov test was used to test the normality of quantitative variables, the χ2, and Fisher’s exact tests to assess differences between proportions, and the Z-test for comparison between proportions, with Bonferroni’s correction.
Comparison between groups was performed using an unpaired t test for variables with normal distribution and homogeneity of variance or the Mann–Whitney U test for those not satisfying these criteria.
Multiple linear regression analysis was adopted to estimate the predictive effect of the independent variables gestational age, sex, birthweight-for-gestational age, and neonatal therapeutic interventions (duration of mechanical ventilation time and oxygen therapy) on pulmonary function (dependent variable). The same instrument of analysis was used to evaluate the influence of respiratory morbidities on pulmonary function.
Pearson’s linear correlation coefficient was adopted to check for correlation between pulmonary function variables and the sum of respiratory morbidities, considering intensity, signal, and direction of correlation and significance.
The numerical variables are represented by measures of central tendency and measures of dispersion. Statistical analysis was performed using the SPSS statistics package 24.0, adopting a 5% significance level.
Results
A total of 123 children were evaluated and divided into two groups: a case group (children born moderate-to-late preterm) with 52 patients and a control group (children born at term) with 71 patients.
Demographic data for the sample are shown in Table 1, indicating homogeneity, except in the case of sex. There was a statistically higher percentage of females (76.9%) in the preterm group compared to the control group (56.3%). The characteristics of the newborns, as well as the main pathologies diagnosed and interventions most frequently used during neonatal care of children in the preterm group, are presented in Table 2.
Three subjects in the case group and one in the control group failed to perform all stages of the assessment properly and were excluded from the IOS analysis. The mean values of oscillometric variables for the baseline pulmonary function of children in the preterm and control group and percentages for the corresponding predicted values are listed in Table 3. No difference was observed between the groups for the variables mentioned. There was also no difference between the mean values of the bronchodilator response variables between the groups, as shown in Table 4.
The lung function measurements were also compared between preterm children with or without respiratory morbidities and no difference was found (p > 0.05).
There was no significant influence of sex, gestational age, birthweight-for-gestational age, duration of invasive mechanical ventilation (IMV), duration of oxygen therapy, and respiratory morbidities on the oscillometric variables (p > 0.05). Of all evaluated children, 61.5% were submitted to supplemental oxygen therapy and 3.8% were intubated and remained on IMV for 7 days, each. The mean time of oxygen therapy was 2.48 days, which varied from 1 to 15 days.
In relation to the response to the bronchodilator, only one child out of 49 (2%) in the preterm group and 2/70 (2.8%) in the control group presented a reduction in R5Hz after bronchodilator use ≥40% (p = 0.999). No child in either the preterm or the control group presented an increase in X5H > 50%, indicating no difference in bronchodilator response in the sample studied.
Nine questionnaires were not answered by the children’s caregivers/guardians. There were no differences between the groups regarding the presence of respiratory morbidities. Nor was any correlation found between lung function and respiratory morbidities taken as a whole (r < 0.19; p > 0.05). The prevalence figures for the most important of these, for each group, are shown in Table 5.
Discussion
It should be noted that this study broke new ground in introducing parameters for impedance of the respiratory system, assessed by means of IOS, in school-age children born moderate-to-late preterm. No differences were found between the means for baseline pulmonary function and percentage predicted values between children enrolled in this study nor for bronchodilator response or long-term morbidities related to premature birth. A number of hypotheses may explain these findings:
-
(a)
Pulmonary plasticity with alveolarization occurs over a longer period of time.
-
(b)
School-age children born moderate-to-late preterm have lung function similar to those born at term. Prematurity, therapeutic interventions, and/or the morbidities presented on birth may have been insufficient in magnitude to impact lung function.
-
(c)
It is possible that the number of participants included was too small to detect aclinically important difference between groups, this being one of the limitations of the study.
Our results contrast with our initial expectations based on the available data, which suggest that children born late preterm may be at risk of decreased lung function in later life. A review of 24 studies of infants born at a gestational age of 32–36 weeks showed that moderate-to-late preterm infants presented more respiratory morbidities than those born at term. These were at times equivalent in magnitude to those found in very premature infants.8 Likewise, a systematic review with meta-analysis has shown that late preterm infants are more likely to require mechanical ventilation and are at greater risk of acute neonatal respiratory dysfunctions.9 Most studies that evaluated pulmonary function in children born preterm have been carried out in very preterm and extremely preterm infants, in moderate-to-late preterm infants during the neonatal period, or in preschool-age children. Few studies in children born moderate-to-late preterm carried out at school-age are available in the literature and those that are available present results that diverge from those of the present study.
Kotecha et al.,20 in a review article, mention that premature children who used IMV and oxygen therapy during neonatal care presented lower pulmonary function values than those not undergoing these therapeutic interventions. The impact of these interventions on lung growth and development and, consequently, on lung function is related to their potential inflammatory effect on lung tissue.21 The percentage for use of noninvasive ventilation in the preterm group was high (46.1%), but that used IMV, which has more commonly been associated with impaired lung function, was only 3.85%. The rate of oxygen use by preterm infants was 65.1%, but the average time of use of this support was brief (2.48 days) and did not influence the pulmonary function measurements obtained.
Er et al.22 carried out IOS in children aged 3–7 years born late preterm and found significantly lower R5 Hz and R5–R20 values compared to those born at term. However, the authors themselves remark that this difference may have been exaggerated by a selection bias present in the preterm and control samples that may have interfered with the extrapolation of results.
Thunqvist et al.,4 in a large-scale cohort study, carried out spirometry in children at 8 years of age and re-evaluated these same children at 16 years of age by way of spirometry and oscillometry. The authors found lower VEF1 and R5 Hz values in those born moderate-to-late preterm compared to those born at term. These effects were greater among males and decreases in R5–R20 and AX were also observed in male children. The fact that this cohort study was initiated ~16 years before any of the children included in the present study had been born makes it difficult to compare the two. There are great differences between the two populations, in terms of technological advances, the development of new treatments, and changes in perinatal care practices, such as the use of less aggressive ventilation strategies in recent years. Less damage to pulmonary function is expected nowadays.23 Although no other studies have found a significant association between sex and lung function, the authors considered the possibility that some differences may have been determined by biological sex, with males being more susceptible to structural alterations and dysfunctions.4 Our study included more female preterm children, yet found no association between sex and pulmonary function.
Most studies investigating bronchodilator response in preterm children have also included those born extremely preterm, with bronchopulmonary dysplasia, or very/extremely low birthweight. Although preterm children with these characteristics exhibited a considerable bronchodilator response, principally among those with bronchopulmonary dysplasia, there are huge disparities in such studies in terms of the clinical profile of the preterm children, gestational age, bronchodilator dose, and the criteria used to evaluate the bronchodilator response, making it impossible to carry out any conclusive comparative analysis. Only two studies included moderate-to-late preterm infants, but these subjects also presented bronchopulmonary dysplasia or birthweight < 1.5 kg and were thus very different from those in our sample, in which there were no cases of bronchopulmonary dysplasia and a lower percentage of children of this weight (19.2%).24,25
The absence of pulmonary dysfunction at school age may indicate pulmonary plasticity and recovery of lung function in the first years of life. Few studies tracked lung function in preterm infants over time or evaluated post-dysfunction recovery ability, but some have found improvement in lung function between 7 and 8 years of age20,26 and point to a potential for such improvement. There is some evidence in the literature to support this idea. Lung and airway cells exhibit a capacity for plasticity and regeneration in response to insults such as infection and exposure to tobacco.27,28,29
The pattern of postnatal lung growth has puzzled many researchers, and postmortem findings suggest that there is great variability in the number of alveoli present in humans, not only at birth but also in childhood.30 The point where alveolar multiplication ends is still not clear. Some believe alveolarization to end ~2–3 years of age, while others defend the hypothesis that new alveoli are formed until at least 8 years of age, and that, thereafter, additional lung growth occurs only by way of growth in size of the alveoli.31,32
Some authors have considered the possibility that pulmonary plasticity with alveolarization may extend over a longer period of time, especially in premature infants. Yammine et al.,31 studying lung function in children born prematurely, have shown that alveolarization continues until at least school age while the bronchioles grow in size until the age of ~22 years.32 Yammine et al. 33 is not in the reference list. Please either provide the complete reference details or delete the citation from text.
Conclusion
In the studied population, pulmonary impedance parameters and the occurrence of respiratory morbidities in school-age children born moderate-to-late preterm were found to be similar to those in children born at term. These findings suggest that pulmonary plasticity with alveolarization may occur up to school age or that prematurity, therapeutic interventions, and/or the morbidities presented at neonatal age may have occurred in an insufficient magnitude to impact lung function.
Change history
15 June 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41390-021-01599-z
References
Carriere, D. et al. Mortality and morbidity of preterm neonates weighing less than 750g: a 2-year retrospective cohort study. Arch. Pediatr. 27, 227–232 (2020).
Vogel, J. P. et al. The global epidemiology of preterm birth. Best. Pract. Res. Clin. Obstet. Gynaecol. 52, 3–12 (2018).
Hee Chung, E., Chou, J. & Brown, K. A. Neurodevelopmental outcomes of preterm infants: a recent literature review. Transl. Pediatr. 9(Suppl.), S3–S8 (2020).
Thunqvist, P. et al. Lung function at 8 and 16 years after moderate-to-late preterm birth: a prospective cohort study. Pediatrics 137, e2015–e2056 (2016).
Haataja, P. et al. Asthma and atopic dermatitis in children born moderately and late preterm. Eur. J. Pediatr. 175, 799–808 (2016).
Moreno-Galdó, A. et al. Recurrent wheezing during the first 3 years of life in a birth cohort of moderate-to-late preterm infants. Pediatr. Allergy Immunol. 31, 124–132 (2020).
Jaiswal, A., Murki, S., Gaddam, P. & Reddy, A. Early neonatal morbidities in late preterm infants. Indian Pediatr. 48, 607–611 (2011).
Colin, A. A., McEvoy, C. & Castile, R. G. Respiratory morbidity and lung function in preterm infants of 32 to 36 weeks’ gestational age. Pediatrics 126, 115–128 (2010).
Teune, M. J. et al. A systematic review of severe morbidity in infants born late preterm. Am. J. Obstet. Gynecol. 205, 374.e1–e9 (2011).
Morata-Alba, J., Romero-Rubio, M. T., Castillo-Corullón, S. & Escribano-Montaner, A. Respiratory morbidity, atopy and asthma at school age in preterm infants aged 32–35 weeks. Eur. J. Pediatr. 178, 973–982 (2019).
Iliodromiti, Z. et al. Acute lung injury in preterm fetuses and neonates: mechanisms and molecular pathways. J. Matern. Fetal Med. Neonatal 26, 1696–1704 (2013).
Beydon, N. et al. An Official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am. J. Respir. Crit. Care Med. 175, 1304–1345 (2007).
Oostveen, E. et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur. Respir. J. 22, 1026–1041 (2003).
King, G. G. et al. Technical standards for respiratory oscillometry. Eur. Respir. J. 55, 1900753 (2020).
Dencker, M. et al. Reference values for respiratory system impedance by using impulse oscillometry in children aged 2–11 years. Clin. Physiol. Funct. Imaging 26, 247–250 (2006).
Malmberg, L. P. et al. Determinants of respiratory system input impedance and bronchodilator response in healthy Finnish preschool children. Clin. Physiol. Funct. Imaging 22, 64–71 (2002).
Smith, H. et al. Forced oscillation technique and impulse oscillometry. In: lung function testing. Eur. Respir. Mon. 31, 72–105 (2005).
Rodrigues, J. et al. Diretrizes para Testes de Função Pulmonar. Provas de Função Pulmonar em Crianças e Adolescentes. J. Pneumol. 28, S207–S221 (2002).
Shin, Y. H. et al. Oscillometric and spirometric bronchodilator response in preschool children with and without asthma. Can. Respir. J. 19, 273–277 (2012).
Kotecha, S. J., Dunstan, F. D. & Kotecha, S. Long term respiratory outcomes of late preterm-born infants. Semin. Fetal Neonatal Med. 17, 77–81 (2012).
Chakraborty, M., McGreal, E. P. & Kotecha, S. Acute lung injury in preterm newborn infants: mechanisms and management. Paediatr. Respir. Rev. 11, 162–170 (2010).
Er, I. et al. Evaluation of lung function on impulse oscillometry in preschool children born late preterm. Pediatr. Int. 58, 274–278 (2016).
Carvalho, C. G., Silveira, R. C. & Procianoy, R. S. Lesão pulmonar induzida pela ventilação em recém-nascidos prematuros. Rev. Bras. Ter. Intensiv. 25, 319–326 (2013).
Fawke, J. et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am. J. Respir. Crit. Care Med. 182, 237–245 (2010).
Kotecha, S. J. et al. Effect of bronchodilators on forced expiratory volume in 1 s in preterm-born participants aged 5 and over: a systematic review. Neonatology 107, 231–240 (2015).
Doyle, L. W. et al. Changes in lung function between age 8 and 14 years in children with birth weight of less than 1,501 g. Pediatr. Pulmonol. 27, 185–190 (1999).
Beers, M. F. & Morrisey, E. E. The three R’s of lung health and disease: repair, remodeling, and regeneration. J. Clin. Invest. 121, 2065–2073 (2011).
Hogan, B. L. et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15, 123–138 (2014).
Spella, M., Lillis, I. & Stathopoulos, G. T. Shared epithelial pathways to lung repair and disease. Eur. Respir. Rev. 26, 170048 (2017).
Mullassery, D. & Smith, N. P. Lung development. Semin. Pediatr. Surg. 24, 152–155 (2015).
Yammine, S. et al. Functional evidence for continued alveolarisation in former preterms at school age? Eur. Respir. J. 47, 147–155 (2016).
Zeman, K. L. & Bennett, W. D. Growth of the small airways and alveoli from childhood to the adult lung measured by aerosol-derived airway morphometry. J. Appl. Physiol. 100, 965–971 (2006).
Author information
Authors and Affiliations
Contributions
F.M.N.A.D.: literature search, data collection, study design, data analysis, manuscript preparation, and manuscript review; E.S.C.S.: study design, data analysis, and manuscript review; P.A.F.M. and L.B.A.: review of data, manuscript preparation, and review. J.A.R. and D.M.P.: study design, data collection, data analysis, and manuscript review. All authors listed made a substantial, direct intellectual contribution to the work, and approved it for publication.E.C.N.H.: literature search, data collection, study design.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement:
The children’s caregivers/guardians were informed about the study and, upon agreeing to participate, signed the informed consent form.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Due to a processing error, the affiliations were incorrect and one affiliation was missing.
Rights and permissions
About this article
Cite this article
Dantas, F.M.N.A., Magalhães, P.A.F., Hora, E.C.N. et al. Lung mechanics and respiratory morbidities in school-age children born moderate-to-late preterm. Pediatr Res 91, 1136–1140 (2022). https://doi.org/10.1038/s41390-021-01538-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01538-y