Abstract
We evaluated possible mediators underlying lifestyle intervention effects on neonatal adiposity, assessed with sum of skinfolds and cord blood leptin. This is a secondary analysis of the DALI study, a randomised controlled trial in nine European countries. Pregnant women with a pre-pregnancy body mass index of ≥29 kg/m2 were randomly assigned to counselling for healthy eating (HE), physical activity (PA), HE&PA combined, or to usual care. We considered five maternal metabolic factors at 24–28 and 35–37 weeks of gestation, and four cord blood factors as possible mediators of the effect of combined HE&PA counselling on neonatal adiposity. From all potential mediators, the intervention only affected cord blood non-esterified fatty acids (NEFA), which was higher in the HE&PA group compared to UC (0.068 (mmol/L), 95% CI: 0.004 to 0.133). Cord blood NEFA did not mediate the HE&PA intervention effects on neonatal sum of skinfolds or cord blood leptin, based on an indirect effect on skinfolds of 0.018 (mm), 95% CI: −0.217 to 0.253 and an indirect effect on leptin of −0.143 (μg/l), 95% CI: −0.560 to 0.273. The Dali study observed reductions in neonatal adiposity in pregnant women with obesity, but we were not able to identify the underlying metabolic pathway.
Similar content being viewed by others
Introduction
Previously, we reported that in women with obesity the DALI (Vitamin D And Lifestyle Intervention for Gestational Diabetes Mellitus (GDM) Prevention) combined healthy eating (HE) and physical activity (PA) intervention was effective in reducing neonatal adiposity, as assessed by the sum of skinfolds and cord blood leptin,1 and also effective in reducing maternal weight gain.2 Although we found that a reduction in sedentary time mediated the intervention effect on neonatal adiposity,1 we do not know which physiological changes account for this effect. In this cohort, several maternal metabolic factors were associated with neonatal adiposity.3,4 Observational studies are useful in proposing theoretical models depicting relationships among a set of exposures and the outcome. More specifically, Lima et al.4 and Shapiro et al.5 published frameworks suggesting how maternal metabolic factors during pregnancy would be associated with neonatal adiposity using mediation models in observational studies. However, only randomised clinical trials can more appropriately assert on causality. Therefore, we aimed to identify possible mediators underlying the DALI intervention effects on neonatal adiposity, assessed with the sum of skinfolds and cord blood leptin as proxy measures.
Methods
Subjects
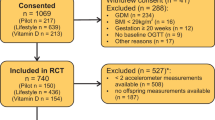
The DALI Study is a randomised controlled trial (ISRCTN70595832), conducted in nine European countries.1,2 Pregnant women with a pre-pregnancy body mass index of ≥29 kg/m2, before 20 weeks of gestation, with a singleton pregnancy, and aged ≥18 years were invited to participate. Exclusions included diagnosis with early gestational diabetes mellitus, pre-existing diabetes, and chronic medical conditions. Local ethics committee approval and written informed consent were obtained.
Randomisation and intervention
Women were randomly assigned to counselling for HE, PA, HE&PA combined, or to usual care (UC). In the intervention groups, participants were assigned to a single coach, with whom they discussed five PA and/or seven HE messages, depending on group allocation, and were advised to keep gestational weight gain <5 kg. Coaching, inspired by motivational interviewing, took place during five face-to-face sessions of 30–45 min each, alternated with up to four optional telephone calls. In the UC group, participants received no DALI interventions.
Primary outcomes
In neonates, triceps, subscapular, supra-iliac, and quadriceps skinfolds were measured within 2 days after birth, and values summed (sum of skinfolds). Venous cord blood samples were taken immediately after delivery and stored at −20 °C or colder until further analysis in the central trial laboratory in Graz, Austria. Leptin concentrations were quantified by solid-phase sandwich enzyme-linked immunosorbent assay (E05–086–96; EIASON, Graz, Austria), according to the manufacturer’s instructions. Analytical sensitivity was 1.0 ng/mL; intra- and inter-assay coefficients of variability (low/high concentrations) were 6.0/6.9 and 11.6/8.7%, respectively.
Potential mediating variables
We considered several maternal metabolic factors at 24–28 and 35–37 weeks of gestation as possible mediators: Stumvoll first and second phase insulin secretion, triglycerides, non-esterified fatty acids (NEFA), and 3-β-hydroxybutyrate. After fasting for 10 h, blood was collected. All the samples were centrifuged and separated aliquots (1000 or 250 μL) placed in microrack tubes and stored at −20 °C or −80 °C in the central trial laboratory, prior to analysis, in Graz, Austria. Note that there were other possible secondary outcomes that could be considered in this study: maternal weight gain, fasting glucose, fasting insulin, and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR). However, Simmons et al. already reported no HE&PA intervention effects on fasting glucose, fasting insulin, and HOMA-IR.2 Although Simmons et al. observed intervention effects on weight gain,2 van Poppel et al. already showed that maternal weight gain did not mediate the HE&PA intervention effects on neonatal sum of skinfolds or cord blood leptin.1
Additionally, we considered cord blood C-peptide, glucose, triglycerides, NEFA, and total cholesterol as potential foetal mediators. Venous cord blood samples were taken immediately after delivery and stored at −20 °C or colder until further analysis in the central trial laboratory in Graz, Austria.
Statistical analysis
We have >80% statistical power to detect intervention effects with effect sizes of f2 = 0.18 considering the number of groups (n = 4), repeated measures (n = 2), and 334 mother–infant pairs included. Structural equation modelling was used for estimating direct effects of the interventions on the secondary outcomes individually since previous publications already reported the intervention effects on the sum of skinfold and leptin.1
For secondary outcomes affected by the HE&PA group, we estimated the indirect (mediated) effects of the intervention via the respective secondary outcome. All the analysis accounted for the cluster structure of the data (individuals nested within countries). Intervention effect analysis on cord blood secondary outcomes were adjusted by mode of delivery and gestational age at birth. Intervention effect analysis on maternal secondary outcomes was adjusted by the respective exposure at <20 weeks of gestation. We accepted 5% type I error.
Results
A total of 436 women were included in the trial. Participants lost to follow-up (n = 102) were not different from those for whom neonatal data were available (n = 334, 50.3% boys). The mean age, in years, was 31.9 ± 5.6 (UC), 32.5 ± 5.3 (HE&PA), 31.4 ± 5.5 (HE), and 31.7 ± 4.9 (PA); pre-pregnancy body mass index, in kg/m2, was 33.7 ± 3.7 (UC), 33.6 ± 3.6 (HE&PA), 34.2 ± 4.6 (HE), and 33.8 ± 3.9 (PA); and, weight gain during pregnancy at 35–37 weeks (kg) was 8.6 ± 4.6 (UC), 6.4 ± 3.9 (HE&PA), 7.6 ± 4.9 (HE), and 8.2 ± 4.9 (PA). Most of the mothers were of European descendent: 89% (UC), 86% (HE&PA), 86% (HE), and 83% (PA). Maternal characteristics were comparable between intervention groups, except for weight gain during pregnancy in which HE&PA showed lower weight gain compared to UC.
From all potential meditating variables, the combined HE&PA intervention only affected cord blood NEFA. More specifically, cord blood NEFA was higher in the HE&PA intervention group compared to UC (0.068, 95% confidence interval (CI): 0.004–0.133; Table 1).
Figure 1 presents the direct effect of the HE&PA intervention on cord blood NEFA besides the intervention effects on neonatal skinfolds and cord blood leptin. In both models, HE&PA intervention affected primary and secondary outcomes. However, cord blood NEFA was not associated with skinfolds or cord blood leptin. Therefore, cord blood NEFA did not mediate the HE&PA intervention effects on neonatal sum of skinfolds or cord blood leptin (Fig. 1). Total (direct plus indirect effect) intervention effects were −1.94 mm (95% CI: −3.59 to −0.30) for sum of skinfolds and 3.87 µg/L (95% CI: −7.55 to −0.18) for cord blood leptin.
Discussion
A counselling intervention for HE&PA in pregnant women with obesity effectively decreased neonatal adiposity. Although findings from observational studies suggest a role of maternal glucose, insulin resistance, and lipid profiles during pregnancy in intrauterine fat accretion,3,4,5 we failed to identify mediators of our intervention effect among those factors measured at multiple time points in pregnancy. Participants in the intervention and control groups were comparable at the start of the intervention. Hence, alternative metabolic pathways must be the key drivers for the intervention effects.
Metabolomics might be needed to find the piece of the puzzle that we are missing.6,7 In a study conducted with 1600 mother–newborn pairs, groups of interrelated amino acid, acylcarnitine, and fatty acid metabolites in both maternal and cord blood were associated with the neonatal sum of skinfolds.6 It is possible that the DALI HE&PA intervention resulted in changes in those groups of metabolites that subsequently led to lower adiposity in the offspring. In addition, we may have to expand the range of candidate mediators beyond conventional metabolites and include effects of the microbiome, exosomes, and others.
It is also possible that, compared to skinfolds, the use of more direct measures of neonatal adiposity, such as air displacement plethysmography, dual-energy X-ray absorptiometry scans, or magnetic resonance imaging would allow more precise estimation of intervention effects. Nevertheless, results remained the same using cord blood leptin, which reflects total foetal fat mass and not only subcutaneous fat, in contrast to skinfold measurements.
Conclusions
We observed reductions in neonatal adiposity resulting from a lifestyle intervention in pregnant women with obesity. Although the results are encouraging, we are not able to identify the metabolic pathways that resulted in lower adiposity level in neonates. Future studies evaluating mediators of intervention effects on neonatal adiposity should incorporate other possible metabolic pathways since the most conventional metabolites do not seem to drive such interventional effects on neonatal adiposity.
Data availability
Deidentified data might be made available upon reasonable request to M.N.M.v.P. (mireille.van-poppel@uni-graz.at).
References
van Poppel, M. N. M. et al. A reduction in sedentary behaviour in obese women during pregnancy reduces neonatal adiposity: the DALI randomised controlled trial. Diabetologia 62, 915–925 (2019).
Simmons, D. et al. Effect of physical activity and/or healthy eating on GDM risk: the DALI Lifestyle Study. J. Clin. Endocrinol. Metab. 102, 903–913 (2017).
Lima, R. et al. Temporal relationships between maternal metabolic parameters with neonatal adiposity in women with obesity differ by neonatal sex: secondary analysis of the DALI study. Pediatr. Obes. 15, e12628 (2020).
Lima, R. A. et al. The importance of maternal insulin resistance throughout pregnancy on neonatal adiposity. Paediatr. Perinat. Epidemiol. 35, 83–91 (2020).
Shapiro, A. L. B. et al. Testing the fuel-mediated hypothesis: maternal insulin resistance and glucose mediate the association between maternal and neonatal adiposity, the Healthy Start study. Diabetologia 58, 937–941 (2015).
Kadakia, R. et al. Maternal metabolites during pregnancy are associated with newborn outcomes and hyperinsulinaemia across ancestries. Diabetologia 62, 473–484 (2019).
Kadakia, R. et al. Cord blood metabolomics: association with newborn anthropometrics and C-peptide across ancestries. J. Clin. Endocrinol. Metab. 104, 4459–4472 (2019).
Acknowledgements
This work was supported by European Union 7th framework (FP7/ 2007–2013) under Grant Agreement no. 242187. In the Netherlands, additional funding was provided by the Netherlands Organisation for Health Research and Development (ZonMw) (grant 200310013). In Poland, additional funding was obtained from Polish Ministry of Science (grants 2203/7, PR/2011/2). In Denmark, additional funding was provided by Odense University Free Research Fund. In Spain, additional funding was provided by CAIBER (1527-B-226). The funders had no role in any aspect of the study beyond funding.
The DALI core investigator group
Rodrigo Antunes Lima1, Gernot Desoye2, David Simmons3,4, Roland Devlieger5, Sander Galjaard5,6, Rosa Corcoy7,8, Juan M. Adelantado7,8, Fidelma Dunne9, Jürgen Harreiter10, Alexandra Kautzky-Willer10, Peter Damm11,12, Elisabeth R. Mathiesen11,12, Dorte M. Jensen13,14,15, Lise-Lotte Andersen13,14,15, Mette Tanvig13,14,15, Annunziata Lapolla16, Maria Grazia Dalfra16, Alessandra Bertolotto17, Ewa Wender-Ozegowska18, Agnieszka Zawiejska18, David J. Hill19, Frank J. Snoek20,21 and Judith G. M. Jelsma22
Author information
Authors and Affiliations
Consortia
Contributions
R.A.L. designed this study, conducted the statistical analysis, contributed to the interpretation of the results, and drafted the manuscript. G.D. and M.N.M.v.P. designed this study, contributed to the interpretation of the results, and drafted the manuscript. All authors, except R.A.L., made substantial contribution to the conception of the DALI Study and acquisition of data. All authors revised the manuscript, contributed to the content, approved the final manuscript as submitted, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Written informed consent were obtained before enrolment of participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lima, R.A., Desoye, G., Simmons, D. et al. Mediators of lifestyle intervention effects on neonatal adiposity: are we missing a piece of the puzzle?. Pediatr Res 91, 522–525 (2022). https://doi.org/10.1038/s41390-021-01450-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01450-5