Abstract
Background
Milk cholesterol concentrations throughout lactation were analyzed, and the relationship between maternal plasma cholesterol and milk cholesterol in various Chinese populations was examined.
Methods
A sub-sample of 1138 lactating women was randomly selected from a large cross-sectional study in China (n = 6481). Milk cholesterol concentrations were determined by HPLC, and concentrations of maternal plasma lipids were determined by an automated biochemical analyzer.
Results
The mean cholesterol concentrations were 200, 171, and 126 mg/L for colostrum, transitional milk, and mature milk, respectively. Cholesterol concentrations differed significantly between stages of lactation (colostrum vs. transitional milk, colostrum vs. mature milk, transitional milk vs. mature milk, all p < 0.001). Concentrations of maternal plasma total cholesterol (TC) (p = 0.02) and low-density lipoprotein cholesterol (LDL-C) (p = 0.03) were significantly associated with milk cholesterol. Milk cholesterol concentrations varied among different ethnicities (Tibetan vs. Hui: 164 vs. 131 mg/L, p = 0.027) but not among different geographic regions.
Conclusions
The concentration of cholesterol in human milk changes dynamically throughout lactation. Milk cholesterol concentrations are significantly associated with maternal plasma concentrations of TC and LDL-C, and milk cholesterol concentrations vary across ethnicities in China.
Impact
-
Concentrations of milk cholesterol were measured in various Chinese populations.
-
Cholesterol concentrations differ significantly between stages of lactation.
-
Maternal plasma total cholesterol and low-density lipoprotein cholesterol are associated with milk cholesterol.
-
Milk cholesterol concentrations vary across ethnicities in China.
Similar content being viewed by others
Introduction
Cholesterol is a structural component of cell membranes and plays critical roles in forming new tissues and organs, particularly the brain. It is incorporated in significant amounts into myelin in the nervous system during brain development in early life and serves as the substrate for biosynthesis of bile acids, lipoproteins, vitamin D, hormones, and oxysterols.1 It is known that dietary cholesterol regulates synthesis and metabolism of endogenous cholesterol and that dietary cholesterol intake is inversely associated with de novo cholesterol biosynthesis by regulating enzymes, such as 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, involved in endogenous cholesterol synthesis.2,3
Human milk contains significant amounts of cholesterol (90–150 mg/L), which is the only exogenous source of cholesterol for exclusively breast-fed infants.4 By contrast, regular infant formulas contain very little cholesterol (0–4 mg/L)4 since bovine milk fat is traditionally removed and replaced with vegetable oils to simulate the human milk fatty acid composition.5,6 It has been documented that the bovine milk fat globule membrane (MFGM) has beneficial effects on infant development,5,7 so MFGM has been added to some infant formulas.8 Since cholesterol is one of the components of MFGM (~7.6 mg/g in MFGM, Arla Foods Ingredients, Viby, Denmark), some infant formulas may contain higher concentrations of milk cholesterol than do regular infant formulas. Accumulating evidence shows that milk cholesterol has short- and long-term effects on cholesterol metabolism and the lipid profile in the offspring. Compared to formula-fed infants, breast-fed infants have higher plasma cholesterol and low-density lipoprotein cholesterol (LDL-C) in early life9,10 and lower plasma cholesterol later in life.11,12,13,14 High plasma cholesterol in infancy may contribute to early cognitive development,15 and lower plasma cholesterol in adulthood may lead to reduced risk of cardiovascular disease.16 Studies using animal models (mouse, rat, and piglet) show that dietary cholesterol promotes brain development in early life by increasing myelination,17 brain weight, and brain cholesterol content.18,19,20
Human milk cholesterol changes dynamically during lactation.21,22 In general, milk cholesterol concentration is highest in colostrum and gradually decreases with lactation progression. Additional factors reported to be related to differences in milk cholesterol concentrations include geographic location,23,24 ethnicity,25 and diet.26 Cholesterol concentrations in mature human milk human range from 101 to 233 mg/L in Australia, Europe, and the USA21,23,27,28,29,30,31; the different milk cholesterol concentrations may be caused by dietary, ethnic, or geographical differences and/or analytical methods. It is known that dietary differences in the proportion of lipid to carbohydrate and the amount of dietary fat alter milk lipid composition.26 In a rabbit study, severe maternal hypercholesterolemia induced by dietary fat and cholesterol resulted in increased milk cholesterol concentration and consequent elevated cholesterol intake by the pups.32 However, dietary fat and cholesterol intake and plasma cholesterol levels were not found to be associated with milk cholesterol concentrations in a human study.27 Although maternal body weight was negatively associated with breast milk cholesterol in a study,29 concentrations of human milk nutrients, such as milk protein, carbohydrate, and minerals, were generally conserved across populations regardless of nutrition status.33,34 Breast milk from Tibetan mothers contains higher fat concentrations compared to other populations.25 Therefore, ethnicity could be a factor that contributes to various milk cholesterol concentrations.
Milk cholesterol concentrations throughout lactation in Chinese populations have not been previously studied. The objectives of the present study were to examine the dynamic change in human milk cholesterol throughout the course of lactation and to assess the relationship between maternal plasma concentration of lipids [total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), LDL-C, and triglycerides (TG)] and breast milk cholesterol concentrations in Chinese populations.
Materials and methods
Study design
This investigation is part of a large-scale human milk composition study in China, which has been described in detail previously.35 A cross-sectional study was conducted in 11 provinces/autonomous regions/municipalities (Beijing, Gansu, Guangdong, Guangxi, Heilongjiang, Inner Mongolia, Shandong, Shanghai, Xinjiang, Yunnan, and Zhejiang) across China between 2011 and 2013. The study sites were located in northern, southern, western, and eastern China. Both inland areas and coastal areas were included in the study.
Subjects
Lactating women within 0–330 days postpartum and their infants were recruited into the study. Some participants were enlisted at birth centers, and the others were recruited from communities at later lactation stages. Besides Han ethnicity, minor ethnicities (Bai, Dai, Hui, Mongolian, Tibetan, Uyghur, and Zhuang) were included. In total, 6481 dyads of self-reported healthy lactating mothers and their infants were recruited in the original study. A subsample of 1138 was randomly sampled from the original study according to lactation stage and study site (Supplementary Table 2). Colostrum, transitional milk, and mature milk were defined as milk within 0–7 days postpartum, milk within 8–14 days postpartum, and milk after 14 days postpartum, respectively. A structured questionnaire was used to collect socio-economic status, demographic status, lifestyle, gestational and parturition information, birth outcome, breastfeeding information, and introduction of complementary foods. A food frequency questionnaire was used to determine food consumption prior to the study. The study followed the principles of Declaration of Helsinki and was approved by the Medical Ethics Committee of the National Institute for Nutrition and Food Safety, Chinese Center for Disease Control and Prevention (CCDC). All study participants signed the consent form before enrollment in the study. The study was registered at Chinese Clinical Trial Registry as ChiCTR-EOC-16009016.
Human milk processing
Human milk was collected using a portable electric breast pump (HNR/X-2108Z, Shantou, Guangdong, China) in the morning (9 a.m. to 11 a.m.). One breast was emptied (full-breast milk) by pumping into a feeding bottle. The milk sample was fully mixed, aliquoted into 15 mL centrifuge tubes, and then stored at ≤−20 °C in a freezer in the field. Samples were subsequently transported to a central laboratory at the National Institute for Nutrition and Health, CCDC, in Beijing and stored in a −80 °C freezer until analysis.
Human milk cholesterol analysis
Human milk cholesterol was analyzed using a high-performance liquid chromatography (HPLC) method, which is the Chinese national standard method for determining cholesterol levels in food items (GB/T 22220-2008: https://www.chinesestandard.net/PDF/English.aspx/GBT22220-2008). In brief, saponification of milk samples was conducted with absolute ethanol and potassium hydroxide. Cholesterol was extracted from the mixture with diethyl ether and petroleum ether. The solvent was then evaporated, and cholesterol was dissolved in methanol for HPLC analysis (Shimadzu LC-20A HPLC system, Shimadzu, Kyoto, Japan). The minimum detection level of the method was 2.6 mg/100 g. The coefficient of variation (CV) of quality control samples was 7.5%.
Blood sample collection and determination of blood lipids
Fasting blood samples (5 mL) were collected from lactating women (n = 402) 30–330 days postpartum in the morning using vacutainer blood collection tubes (BD Diagnostics, Franklin Lakes, NJ). Plasma lipids (TC, HDL-C, LDL-C, and TG) were analyzed with an automated biochemical analyzer (Hitachi 7600, Hitachi, Tokyo, Japan). The CVs of TC, HDL-C, LDL-C, and TG were 0.80%, 2.70%, 3.18%, and 1.53% for high-level quality control serum and 0.43%, 9.53%, 4.64%, and 3.51% for low-level quality control serum (Wako Pure Chemical Industries, Ltd., Osaka, Japan), respectively.
Statistical analysis
Descriptive statistics were computed for both continuous variables and categorical variables. All continuous variables [i.e., cholesterol concentration, maternal age, and maternal body mass index (BMI)] were tested for normality. Logarithmic transformation was applied to non-normal distribution variables (i.e., milk cholesterol concentration) for bivariate and multivariate analyses. Mean and standard deviation were used for symmetric variables after excluding outliers. Analysis of variance was used for comparisons of the mean cholesterol concentration of ≥3 groups. The Tukey method was used for adjusting multiple comparisons. The general linear model was used to examine the relationship between milk cholesterol and other factors after controlling for the lactation stage. All analyses were conducted with SAS 9.4 release (SAS Inc., Cary, NC) and GraphPad Prism 8.2.1 (GraphPad Software, San Diego, CA).
Results
Characteristics of subjects and samples
Experimental design and information regarding milk sample collection are shown in Fig. 1. Available data and general characteristics of subjects selected are shown in Table 1. Mean maternal age of all subjects was 26.6 years, and mean pre-pregnant maternal BMI was 22.8 kg/m2. About 14, 15, and 70% of the milk samples were colostrum, transitional milk, and mature milk, respectively. Length for age and weight for length of the studied infants approached the World Health Organization growth standard reference population, Z score of 0 (Table 1).
Milk cholesterol concentrations during lactation
The mean cholesterol concentration was 200, 171, and 126 mg/L for colostrum, transitional milk, and mature milk, respectively. As shown in Fig. 2a, cholesterol concentrations at the three stages of lactation differed significantly from one another (colostrum vs. transitional milk, colostrum vs. mature milk, transitional milk vs. mature milk, all p < 0.001). The cholesterol concentration declined gradually over time from colostrum through the first month, and it reached a plateau after the first month (Fig. 2b).
a Concentrations of cholesterol at three lactation stages. Colostrum (n = 161), transitional milk (n = 177), and mature milk (n = 799). b Milk cholesterol concentrations during lactation. 0–7 days (n = 161), 8–10 days (n = 80), 11–13 days (n = 72), 14–16 days (n = 83), 17–30 days (n = 125), 31–90 days (n = 177), 91–150 days (n = 157), 151–240 days (n = 152), and 241–330 days (n = 130). Bars without a common letter are significantly different (p < 0.05).
After controlling for lactation stage and ethnicity, milk cholesterol concentration was negatively associated with expressed full-breast milk volume (p < 0.001). For every 10 g increase in milk amount, the cholesterol concentration decreased by 1.98 mg/L.
Milk cholesterol concentration was not associated with pre-pregnant BMI (p = 0.82), household income (p = 0.59), number of pregnancy (p = 0.57), length of gestation (p = 0.74), consumption of dietary meat or organ meat (p = 0.39), dairy consumption (p = 0.97), egg consumption (p = 0.52), or nut consumption (p = 0.10). After adjusting for lactation stage and ethnicity, maternal occupation (p = 0.44), delivery mode (p = 0.18), total time of physical activity (p = 0.22), and total time of sleeping (p = 0.45) were also not related to milk cholesterol nor were preterm delivery or infant sex associated with milk cholesterol (p = 0.63 and p = 0.24, respectively).
Concentrations of maternal plasma lipids and relationship between plasma lipid concentrations and milk cholesterol concentrations
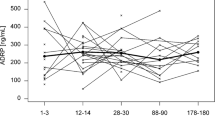
As shown in Fig. 3a, maternal plasma TC, HDL-C, LDL-C, and TG were measured. Plasma TC (p = 0.02) and LDL-C (p = 0.03) are significantly related to the concentration of mature milk cholesterol (collected from 30 to 330 days postpartum). For every 100 mg/L that plasma TC increased and for every 100 mg/L that plasma LDL-C increased, milk cholesterol concentration increased by 1.01 times (Fig. 3b, c). The relationship between human milk cholesterol and plasma TC was marginally significant for early mature milk (30–180 days postpartum, beta = 0.001, p = 0.06) and for late mature milk (180–330 days postpartum, beta = 0.002, p = 0.06) (Supplementary Figs. 1 and 2). However, maternal plasma HDL-C (p = 0.68) and TG (p = 0.42) were not associated with milk cholesterol concentration.
Concentrations of maternal plasma TC, LCL-C, HDL-C, and TG were measured in plasma samples collected from different ethnicities (Fig. 4a, b) and geographical regions (Fig. 4c, d). Since plasma samples were not collected in Xinjiang, where Uyghurs mainly live, no results are available for Uyghurs. Concentrations of plasma TC and LDL-C varied in samples collected from different ethnicities and geographic regions (Fig. 4). Maternal plasma TC (p < 0.01), LDL-C (p < 0.01), and HDL-C (p < 0.01), but not TG (p = 0.66), were associated with ethnicity. Similarly, maternal plasma lipid concentration TC (p < 0.01), LDL-C (p < 0.01), and HDL-C (p = 0.02), but not TG (p = 0.12), correlated with geographical region.
Milk cholesterol concentrations among ethnicities and geographical regions in China
As shown in Fig. 5, milk from Tibetan mothers displayed the highest concentration of milk cholesterol. After controlling for lactation stage, expressed volume of full-breast milk, and ethnicity, the cholesterol concentration was significantly greater in milk from Tibetan mothers than that from Hui mothers (164 vs. 131 mg/L, p = 0.027; Fig. 5). The milk cholesterol concentrations of other populations did not differ, such as Han vs. Hui (145 vs. 129 mg/L, p = 0.55) and Tibetan vs. Zhuang (164 vs. 137 mg/L, p = 0.29). The milk cholesterol difference between Tibetan and Hui mothers was not caused by a difference in maternal serum lipids since no differences in maternal lipid concentrations were found between them (TC: Tibetan vs. Hui = 139.8 vs. 149.1, p > 0.99; LDL-C: Tibetan vs. Hui = 89.1 vs. 95.8 mg/L, p > 0.99; HDL-C: Tibetan vs. Hui = 50.8 vs. 55.9 mg/L, p > 0.99; and TG: Tibetan vs. Hui = 57.1 vs. 74.6 mg/L, p > 0.99). In addition, geographical region was not significantly related to milk cholesterol concentration (p = 0.33).
Discussion
Milk cholesterol concentrations change dynamically throughout lactation. The mean milk cholesterol concentration in these Chinese populations was 200, 171, and 126 mg/L for colostrum, transitional milk, and mature milk, respectively. Cholesterol concentration decreased significantly during the first month postpartum and reached a plateau after that, findings consistent with previous studies.21,28 Most studies have found that milk cholesterol decreases from colostrum to mature milk; one study showed that milk cholesterol dropped by about 50% from colostrum to mature milk (6 months).31 However, another study found that the milk cholesterol level slightly increased from 2 months (88 milk samples) to 6 months (22 milk samples).36 Our study showed that milk cholesterol concentration declined significantly during the first month of lactation and that milk cholesterol in mature milk is about 60% of that in colostrum. After the first month postpartum, the milk cholesterol level tends to be stable (Fig. 2b). Milk lipids are packaged and secreted by a unique mechanism. The MFGM of the milk fat globule consists of a triple phospholipid and cholesterol layer with incorporated proteins and glycoproteins, which surrounds interior droplets of triacylglycerol.5 As lactation stage progresses from colostrum to mature human milk, globule triacylglycerol content increases,37 the globule diameter increases (from 3.8 µm in colostrum to 5.1 µm in mature milk),38 and the number of globules decreases.39 Consequently, a reduction in total globule surface area is accompanied by a reduced level of cholesterol in breast milk with advancing lactation. Results from the current study reflect this trend of milk cholesterol (Fig. 2a). The endogenous cholesterol synthesis is regulated by oral intake of dietary cholesterol,4,40,41 so the de novo synthesis rate of cholesterol increases in breast-fed infants as the milk cholesterol decreases with lactation progression (4–11 months).42 In summary, the impact of dietary (milk) cholesterol on infant cholesterol intake and endogenous production varies throughout lactation stages.
Large variations of milk cholesterol concentrations have been reported previously. Studies in Germany, Spain, the UK, and the USA show cholesterol concentrations in colostrum ranging from 138 to 313 mg/L.31,43 Cholesterol concentrations in mature milk vary between 101 and 233 mg/L in Australia, Germany, Italy, Poland, Spain, and the USA.21,23,27,28,29,30,31 Mean colostrum cholesterol and mean mature milk cholesterol concentrations in the current study are within these ranges but closer to lower end of these ranges. Across these studies, cholesterol concentrations varied significantly; a 2–3-fold difference was seen between the lower end and the upper end of the range.31 Differences may result from variations in the analytical techniques used in those studies.21,28,31 Enzymatic spectrophotometric and colorimetric assays, which are not as specific as chromatographic methods, usually generate higher concentrations of cholesterol than do chromatographic methods.28,31 A study showed that the result from a HPLC method was 20% lower than those from enzymatic–spectrophotometric methods.31
The current study shows that maternal plasma TC and LDL-C are positively correlated with milk cholesterol in Chinese populations. Milk cholesterol is primarily from maternal serum and originates from maternal preformed stores, maternal diet, or de novo synthesis in either liver or mammary gland epithelial cells.44 Some studies have shown that maternal plasma cholesterol levels are not correlated with milk cholesterol in normal subjects.27,45 However, one study found that hypercholesterolemic mothers had higher levels of breast milk cholesterol.23 Different results may be due to small variations in maternal plasma cholesterol and small sample sizes (n = 10–14).27,45 Our study had >400 samples (n = 402) and therefore offers an opportunity to assess the relationship between variable plasma cholesterol and breast milk cholesterol. Since no correlation was found between dietary consumption of meat, organ meat, dairy products, and eggs, the varying storage of cholesterol and endogenous cholesterol biosynthesis likely contribute to the correlation between maternal plasma TC and LDL-C levels and milk cholesterol in Chinese populations.
Higher cholesterol was detected in milk from Tibetan mothers compared with milk from Hui mothers, even though they live in the same geographical region. Since no differences in maternal plasma lipid concentrations were observed in Tibetan and Hui mothers and diet was not correlated with milk cholesterol, Tibetan mothers may have higher endogenous cholesterol biosynthesis and more cholesterol storage than Hui mothers. Consistent with our results, milk from Tibetan mothers contains a higher concentration of fat compared to milk from mothers of other ethnicities.25 Tibetans have lived in the Tibetan Plateau for thousands of years where the partial pressure of oxygen is low and ultraviolet radiation is high. To adjust to these extreme living conditions, Tibetans have evolved unique gene polymorphisms, as reported previously.46 Tibetans may have unique gene variants of enzymes involved in de novo cholesterol biosynthesis, which may lead to higher milk cholesterol. Similarly, in our previous study, milk samples from Tibetan mothers contained the lowest lactoferrin concentration compared to milk samples from mothers in other populations.47 Compared to people living in other regions in China, Tibetans may have higher consumption of butter due to consumption of traditional Tibetan tsamba and butter/milk tea.48 Although it has been reported that the profile of milk lipid and fatty acids is altered by elevated dairy fat intake in lactating women,49,50 the current study did not find an association between breast milk cholesterol levels and dairy consumption or meat consumption (both p > 0.9). The variation in milk cholesterol concentrations may differently affect infant development. Thus a follow-up study to investigate short- and long-term impact of various milk cholesterol concentrations on infant development in these Chinese populations would be valuable.
A limitation of the current study is the cross-sectional study design, which may not provide a causal relationship for the correlation between maternal serum cholesterol and breast milk cholesterol. Although lactation stage was related to milk cholesterol, this study cannot fully rule out inter-subject variation since only one milk sample was available from each mother in the cross-sectional design.
In summary, breast milk cholesterol decreased significantly from colostrum through transitional milk to mature milk and was relatively stable after 1 month of lactation. Maternal serum cholesterol was positively correlated with breast milk cholesterol. Different milk cholesterol concentrations may affect infant development, particularly lipid metabolism and cognitive development. Therefore, a follow-up study is needed to investigate the effect of milk cholesterol on infant development.
References
Cerqueira, N. M. et al. Cholesterol biosynthesis: a mechanistic overview. Biochemistry 55, 5483–5506 (2016).
Dimova, L. G., Lohuis, M. A. M., Bloks, V. W., Tietge, U. J. F. & Verkade, H. J. Milk cholesterol concentration in mice is not affected by high cholesterol diet- or genetically-induced hypercholesterolaemia. Sci. Rep. 8, 8824 (2018).
Berger, S., Raman, G., Vishwanathan, R., Jacques, P. F. & Johnson, E. J. Dietary cholesterol and cardiovascular disease: a systematic review and meta-analysis. Am. J. Clin. Nutr. 102, 276–294 (2015).
Koletzko, B. Human milk lipids. Ann. Nutr. Metab. 69, 28–40 (2016).
Timby, N., Domellof, M., Lönnerdal, B. & Hernell, O. Supplementation of infant formula with bovine milk fat globule membranes. Adv. Nutr. 8, 351–355 (2017).
Zou, L., Pande, G. & Akoh, C. C. Infant formula fat analogs and human milk fat: new focus on infant developmental needs. Annu. Rev. Food Sci. Technol. 7, 139–165 (2016).
Li, F. et al. Improved neurodevelopmental outcomes associated with bovine milk fat globule membrane and lactoferrin in infant formula: a randomized, controlled trial. J. Pediatr. 215, 24–31 e28 (2019).
Brink, L. R. et al. Omics analysis reveals variations among commercial sources of bovine milk fat globule membrane. J. Dairy Sci. 103, 3002–3016 (2020).
Friedman, G. & Goldberg, S. J. Concurrent and subsequent serum cholesterol of breast- and formula-fed infants. Am. J. Clin. Nutr. 28, 42–45 (1975).
Shamir, R. et al. Serum levels of bile salt-stimulated lipase and breast feeding. J. Pediatr. Endocrinol. Metab. 16, 1289–1294 (2003).
Owen, C. G. et al. Does initial breastfeeding lead to lower blood cholesterol in adult life? A quantitative review of the evidence. Am. J. Clin. Nutr. 88, 305–314 (2008).
Singhal, A., Cole, T. J., Fewtrell, M. & Lucas, A. Breastmilk feeding and lipoprotein profile in adolescents born preterm: follow-up of a prospective randomised study. Lancet 363, 1571–1578 (2004).
Hui, L. L. et al. Breastfeeding in infancy and lipid profile in adolescence. Pediatrics 143, e20183075 (2019).
Plancoulaine, S. et al. Infant-feeding patterns are related to blood cholesterol concentration in prepubertal children aged 5-11 y: the Fleurbaix-Laventie Ville Sante study. Eur. J. Clin. Nutr. 54, 114–119 (2000).
Elias, P. K., Elias, M. F., D’Agostino, R. B., Sullivan, L. M. & Wolf, P. A. Serum cholesterol and cognitive performance in the Framingham Heart Study. Psychosom. Med. 67, 24–30 (2005).
Rueda, R. The role of complex lipids in attaining metabolic health. Curr. Cardiovasc. Risk Rep. 8, 371 (2014).
Haque, Z. U. & Mozaffar, Z. Importance of dietary cholesterol for the maturation of mouse brain myelin. Biosci. Biotechnol. Biochem. 56, 1351–1354 (1992).
Schoknecht, P. A. et al. Dietary cholesterol supplementation improves growth and behavioral response of pigs selected for genetically high and low serum cholesterol. J. Nutr. 124, 305–314 (1994).
Boleman, S. L. et al. Pigs fed cholesterol neonatally have increased cerebrum cholesterol as young adults. J. Nutr. 128, 2498–2504 (1998).
Scholtz, S. A., Gottipati, B. S., Gajewski, B. J. & Carlson, S. E. Dietary sialic acid and cholesterol influence cortical composition in developing rats. J. Nutr. 143, 132–135 (2013).
Picciano, M. F., Guthrie, H. A. & Sheehe, D. M. The cholesterol content of human milk. A variable constituent among women and within the same woman. Clin. Pediatr. 17, 359–362 (1978).
Hamosh, M., Bitman, J., Wood, L., Hamosh, P. & Mehta, N. R. Lipids in milk and the first steps in their digestion. Pediatrics 75, 146–150 (1985).
Mellies, M. J., Burton, K., Larsen, R., Fixler, D. & Glueck, C. J. Cholesterol, phytosterols, and polyunsaturated/saturated fatty acid ratios during the first 12 months of lactation. Am. J. Clin. Nutr. 32, 2383–2389 (1979).
Hamdan, I. J. A. et al. Sterols in human milk during lactation: bioaccessibility and estimated intakes. Food Funct. 9, 6566–6576 (2018).
Quinn, E. A., Diki Bista, K. & Childs, G. Milk at altitude: human milk macronutrient composition in a high-altitude adapted population of Tibetans. Am. J. Phys. Anthropol. 159, 233–243 (2016).
Neville, M. C. & Picciano, M. F. Regulation of milk lipid secretion and composition. Annu. Rev. Nutr. 17, 159–183 (1997).
Potter, J. M. & Nestel, P. J. The effects of dietary fatty acids and cholesterol on the milk lipids of lactating women and the plasma cholesterol of breast-fed infants. Am. J. Clin. Nutr. 29, 54–60 (1976).
Clark, R. M., Fey, M. B., Jensen, R. G. & Hill, D. W. Desmosterol in human milk. Lipids 18, 264–266 (1983).
Kamelska, A. M., Pietrzak-Fiecko, R. & Bryl, K. Variation of the cholesterol content in breast milk during 10 days collection at early stages of lactation. Acta Biochim. Pol. 59, 243–247 (2012).
Alvarez-Sala, A., Garcia-Llatas, G., Barbera, R. & Lagarda, M. J. Determination of cholesterol in human milk: an alternative to chromatographic methods. Nutr. Hosp. 32, 1535–1540 (2015).
Hamdan, I. J. A., Sanchez-Siles, L. M., Matencio, E., Garcia-Llatas, G. & Lagarda, M. J. Cholesterol content in human milk during lactation: a comparative study of enzymatic and chromatographic methods. J. Agric. Food Chem. 66, 6373–6381 (2018).
Whatley, B. J., Green, J. B. & Green, M. H. Effect of dietary fat and cholesterol on milk composition, milk intake and cholesterol metabolism in the rabbit. J. Nutr. 111, 432–441 (1981).
Ballard, O. & Morrow, A. L. Human milk composition: nutrients and bioactive factors. Pediatr. Clin. North Am. 60, 49–74 (2013).
Butts, C. A. et al. Human milk composition and dietary intakes of breastfeeding women of different ethnicity from the Manawatu-Wanganui region of New Zealand. Nutrients 10, 1231 (2018).
Yin, S. A. & Yang, Z. Y. An on-line database for human milk composition in China. Asia Pac. J. Clin. Nutr. 25, 818–825 (2016).
Kallio, M. J., Siimes, M. A., Perheentupa, J., Salmenpera, L. & Miettinen, T. A. Cholesterol and its precursors in human milk during prolonged exclusive breast-feeding. Am. J. Clin. Nutr. 50, 782–785 (1989).
Bitman, J., Wood, L., Hamosh, M., Hamosh, P. & Mehta, N. R. Comparison of the lipid composition of breast milk from mothers of term and preterm infants. Am. J. Clin. Nutr. 38, 300–312 (1983).
Wei, W. et al. Phospholipid composition and fat globule structure I: comparison of human milk fat from different gestational ages, lactation stages, and infant formulas. J. Agric. Food Chem. 67, 13922–13928 (2019).
Michalski, M. C., Briard, V., Michel, F., Tasson, F. & Poulain, P. Size distribution of fat globules in human colostrum, breast milk, and infant formula. J. Dairy Sci. 88, 1927–1940 (2005).
Jones, P. J. et al. Dietary cholesterol feeding suppresses human cholesterol synthesis measured by deuterium incorporation and urinary mevalonic acid levels. Arterioscler. Thromb. Vasc. Biol. 16, 1222–1228 (1996).
Wong, W. W., Hachey, D. L., Insull, W., Opekun, A. R. & Klein, P. D. Effect of dietary cholesterol on cholesterol synthesis in breast-fed and formula-fed infants. J. Lipid Res. 34, 1403–1411 (1993).
Bayley, T. M. et al. Longer term effects of early dietary cholesterol level on synthesis and circulating cholesterol concentrations in human infants. Metabolism 51, 25–33 (2002).
Hibberd, C. M., Brooke, O. G., Carter, N. D., Haug, M. & Harzer, G. Variation in the composition of breast milk during the first 5 weeks of lactation: implications for the feeding of preterm infants. Arch. Dis. Child. 57, 658–662 (1982).
Long, C. A., Patton, S. & McCarthy, R. D. Origins of the cholesterol in milk. Lipids 15, 853–857 (1980).
Mellies, M. J. et al. Effects of varying maternal dietary cholesterol and phytosterol in lactating women and their infants. Am. J. Clin. Nutr. 31, 1347–1354 (1978).
Qi, G., Yin, S., Zhang, G. & Wang, X. Genetic and epigenetic polymorphisms of eNOS and CYP2D6 in mainland Chinese Tibetan, Mongolian, Uygur, and Han populations. Pharmacogenomics J 20, 114–125 (2019).
Yang, Z. et al. Concentration of lactoferrin in human milk and its variation during lactation in different Chinese populations. Nutrients 10, 1235 (2018).
Peng, W., Liu, Y., Liu, Y., Zhao, H. & Chen, H. Major dietary patterns and their relationship to obesity among urbanized adult Tibetan pastoralists. Asia Pac. J. Clin. Nutr. 28, 507–519 (2019).
Mohammad, M. A., Sunehag, A. L. & Haymond, M. W. Effect of dietary macronutrient composition under moderate hypocaloric intake on maternal adaptation during lactation. Am. J. Clin. Nutr. 89, 1821–1827 (2009).
Yahvah, K. M. et al. Elevated dairy fat intake in lactating women alters milk lipid and fatty acids without detectible changes in expression of genes related to lipid uptake or synthesis. Nutr. Res. 35, 221–228 (2015).
Acknowledgements
The authors appreciate Judith Hills for critical reading of the manuscript. The authors would like to thank all the mothers who attended this study and field workers who conducted the milk collections. This study was supported by Beijing Natural Science Foundation (S160002), the National Key R&D Program of China (2017YFD0400601), and the National High Technology Research and Development Program of China (863 Program) (2010AA023004).
Author information
Authors and Affiliations
Contributions
Z.Y., R.J., and B.L. drafted the manuscript; H.L. conducted the laboratory analyses; S.Y., Z.Y., J.L., J.W., and Y.D. supervised the field work; X.P., S.J., Y.B., H.Z., and S.W. did the sample selection; S.Y., Z.Y., and J.L. designed the study and are responsible for final content. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent
All study participants signed the consent form.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Yang, Z., Jiang, R., Li, H. et al. Human milk cholesterol is associated with lactation stage and maternal plasma cholesterol in Chinese populations. Pediatr Res 91, 970–976 (2022). https://doi.org/10.1038/s41390-021-01440-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01440-7
This article is cited by
-
Network analysis of the proteome and peptidome sheds light on human milk as a biological system
Scientific Reports (2024)