Abstract
Background
Non-invasive prenatal screening (NIPS) has fundamentally changed the screening process for Down syndrome (DS). Rates of complex congenital heart defects (CHD) have decreased in international studies but whether these shifts exist in the US is unknown.
Methods
Encounters for neonates with DS from 2007 to 2018 were obtained from the Pediatric Health Information System database. CHD were categorized as complex CHD, atrioventricular septal defects (AVSD), ventricular septal defects (VSD), and tetralogy of Fallot (TOF). Comparisons were made between pre-NIPS era (2007–2010) vs. post-NIPS era (2014–2018) and between states with low vs. high access to pregnancy termination as described by the Guttmacher Institute.
Results
Among 9122 patients, 6% had complex CHD, 22% had an AVSD, 22% had a VSD, and 4% had TOF. No difference in proportions of CHD was seen between eras. A small difference was observed in the proportion of AVSD between states with low vs. high access to pregnancy termination (23 vs. 17%, p < 0.001).
Conclusions
The proportion of CHD in patients with DS appears to be stable despite widespread adoption of NIPS in the US. Variations were observed between states with low vs. high access to pregnancy termination. Population based studies are needed to fully evaluate the current epidemiology of CHD in DS.
Impact
-
Through investigation of the Pediatric Health Information System database, this study assesses contemporary epidemiology of congenital heart disease among patients with Down syndrome.
-
It has been suggested that improved prenatal screening for Down syndrome has altered the cardiac phenotype in international populations. Whether a similar shift also exists in the United States is unknown.
-
In a contemporary United States cohort, a shift in the proportion or type of heart defects over the past decade was not observed. Regional differences in the proportion of heart defects were seen and may be due to differential access to prenatal care.
Similar content being viewed by others
Introduction
Down syndrome (DS) is the most common and most viable of the human trisomic syndromes, occurring in approximately 1 per 700 live births in the United States.1 Patients with DS have an increased risk for associated congenital anomalies, including congenital heart defects (CHD), which can lead to increased morbidity, mortality, and health care costs.2,3,4,5,6
Prenatal aneuploidy screening is offered to all pregnant women in the United States, which typically involves a combination of ultrasound measurements and maternal serum biomarker levels. These tests yield a sensitivity of 69–87% (up to 96% sensitivity for serial screening methods) and a screen-positive rate of 5%.7,8,9 It is recommended that positive screens are followed by confirmatory testing through invasive methods, such as chorionic villus sampling or amniocentesis.10 However, beginning in 2011, screening for DS with non-invasive prenatal screening (NIPS) became widely available with increasing adoption in clinical practice.11 This test carries a high sensitivity and low false positive rate for detecting DS (99.2 and 0.5%, respectively, in meta-analysis).12,13,14,15 Once a prenatal diagnosis of DS is made, fetal echocardiography is recommended to provide anticipatory guidance to families regarding coexistent CHD.16
The rate of pregnancy termination following a fetal diagnosis of DS varies between regions, and the impact of NIPS on contemporary termination rates in other countries has been mixed.17 Large-scale population studies from Europe have shown that the incidence of any CHD in DS has remained constant over the past several decades.18 There is also data to suggest a recent phenotypic shift away from so-called “complex” CHD presumably due to an increase in the rate of selective termination in this population.19 Whether these shifts exist in a contemporary cohort in the United States is unknown and the impact of widespread adoption of NIPS on CHD phenotype in patients with DS has not been studied.20
The first aim of this study is to evaluate the proportion of CHD in a contemporary nationwide cohort of live-born neonates with DS and assess the impact of widespread adoption of NIPS. The second aim of the study is to assess for regional variations in DS phenotype based on access to pregnancy termination.
Methods
Data source
A nationwide clinical registry for birth defects does not exist in the US. As an alternative, we used a high-fidelity administrative database. Data for this study were obtained from the Pediatric Health Information System (PHIS), an administrative database that contains inpatient, emergency department, ambulatory surgery, and observation encounter-level data from 51 not-for-profit, tertiary care pediatric hospitals in the United States. These hospitals are affiliated with the Children’s Hospital Association (Lenexa, KS). Data quality and reliability are assured through a joint effort between the Children’s Hospital Association and participating hospitals. Portions of the data submission and data quality processes for the PHIS database are managed by Truven Health Analytics (Ann Arbor, MI). For the purposes of external benchmarking, participating hospitals provide discharge/encounter data, including demographics, diagnoses, and procedures. Data are de-identified at the time of data submission, and data are subjected to a number of reliability and validity checks before being included in the database. For this study, data from all 51 hospitals was included. The study was reviewed by the Nationwide Children’s Hospital Institutional Review Board (IRB) and determined not to involve human subjects under 45 CFR part 46.102(f), therefore IRB evaluation was waived.
Study population
Diagnostic codes from the International Classification of Diseases, Ninth Edition (ICD-9) and Tenth Edition (ICD-10) were used for identification purposes. ICD-9 codes were exclusively used up to 10/2015 followed by exclusive use of ICD-10 codes. Patients with DS who were admitted to participating hospitals at 30 days of age or less and had a discharge date between 1 January 2007 and 31 March 2018 were included in the analysis. Cardiac defects were categorized into four mutually exclusive groups based on diagnostic codes. Complex cardiac defects included single ventricle disease and other cardiac defects commonly requiring neonatal repair (such as truncus arteriosus). The atrioventricular septal defect (AVSD) category included all patients with AVSD who were not already categorized as having complex defects. The tetralogy of Fallot (TOF) category included all patients with TOF who were not already categorized as either complex defects or AVSD and the ventricular septal defect (VSD) category included all patients with VSD who were not already included in a previous category. These diagnosis categories were felt to hold clinical significance for patients and families of patients with DS as most require congenital heart surgery within the first year of life, have little-to-no diagnostic variation between institutions, have minimal diagnostic uncertainty (compared with atrial septal defect which has diagnostic overlap with patent foramen ovale in the newborn period), and do not represent transitional physiology (such as a patent ductus arteriosus). A detailed description of the included ICD-9 and ICD-10 codes are listed in the supplement.
To assess regional variations, we grouped states into three categories based on the Guttmacher Institute’s assessment of access to pregnancy termination: low access, moderate access, and high access to pregnancy termination.21 The classification system measures 12 state-level polices affecting access to pregnancy termination. Further details about the classification system are available elsewhere.22 The high-access states included California, New York, Oregon, and Washington. Low-access states included Alabama, Arizona, Arkansas, Indiana, Kentucky, Louisiana, Mississippi, Missouri, Nebraska, North Carolina, North Dakota, Ohio, Oklahoma, Pennsylvania, South Carolina, South Dakota, Tennessee, Texas, Utah, Virginia, and Wisconsin.
Statistical analysis
The cohort was divided into approximately equal eras: 2007–2010 (pre-NIPS era), 2011–2013 (transitional era), and 2014–2018 (post-NIPS era). Data were tabulated as n (%) and comparisons were made using the chi-square test between the pre-NIPS and post-NIPS eras. The cohort was then stratified by access to pregnancy termination and proportions of CHD were compared again between pre-NIPS and post-NIPS eras. Finally, proportions of CHD were examined over the entire time period and comparisons were made between low-access and high-access states, the classification of which was informed by data from the Guttmacher Institute.
A p value < 0.05 was considered statistically significant. Statistical analyses were performed using STATA version 11 (STATA Corp., TX, USA). Descriptive statistics were presented as medians (interquartile ranges) for continuous variables and frequency counts and percentages for categorical variables. Chi-square and t tests were used to examine the change in defect proportions between groups.
Results
A total of 9122 patients were included in the study. Nearly all demographic data were complete with the exception of gestational age (missing in 2054 encounters, 29%) and birth weight (missing in 685 encounters, 8%). Demographics and clinical characteristics of the cohort are shown in Table 1. The majority of patients were male (4963, 54%), white (5669, 62%), non-Latino (5227, 57%), and had a government payor (4742, 52%). Most patients were born at term with a median birth weight of 2.9 kg. The most common associated (non-cardiac) congenital abnormality was duodenal atresia/stenosis occurring in 12% of the cohort.
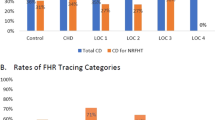
Figure 1 demonstrates the proportions of CHD by year. No changes were observed in any CHD group over the study period. When comparing proportions of CHD in the pre-NIPS era and in the post-NIPS era, no changes were observed (complex CHD p = 0.59, AVSD p = 0.14, TOF p = 0.87, VSD p = 0.23, Table 2). We then stratified the cohort by access to pregnancy termination (Fig. 2). A decrease in the proportion with CHD (including complex CHD, AVSD, TOF, and VSD) was observed in states with high access to pregnancy termination although this did not reach statistical significance (p = 0.22).
A decrease in the proportion of neonates with Down syndrome was observed in states with high access to pregnancy termination although this did not reach statistical significance. The comparisons include patients with complex congenital heart defects, atrioventricular septal defects, tetralogy of Fallot, and ventricular septal defects between pre-non-invasive prenatal screening era (pre-NIPS; 2007–2010) and post-NIPS era (2014–2018).
Finally, with knowledge that there were no temporal changes in the proportions of CHD among the cohort, we compared the proportion of CHD between low and high pregnancy termination access states over the entire study period. Table 3 demonstrates that, over the complete study period, there were no significant differences in the proportions of CHD among states with low vs. high access to pregnancy termination except for AVSD. A lower proportion of AVSD was observed in states with high access to pregnancy termination (p < 0.001).
Discussion
Our study suggests that the proportion of complex CHD, AVSD, TOF, and VSD among live-born neonates with DS has not substantially changed over the past decade in a contemporary US cohort. This is in contrast to the study by Bergstrom and colleagues, which detected a shift in the Swedish CHD phenotype away from complex CHD by nearly 40% over a 20-year period in a national birth registry. It should be noted, however, that their study included AVSD in the complex CHD group, which makes direct comparisons difficult. Their definition of complex CHD was quite broad and whether or not the shift seen in their study was driven by a smaller subset (such as single ventricle disease) is unknown. This is why we chose to separate the AVSD group from the other complex CHD in the present study. Bergstorm and colleagues speculate that the shift they observed was due to increased prenatal diagnosis and pregnancy termination in fetuses with complex CHD and a simultaneous increase in diagnostic sensitivity of echocardiography leading to increased identification of lesions of limited clinic impact (such as isolated atrial septal defect or VSD). Another population registry study by Pfitzer and colleagues in Germany also provides evidence of a shifting phenotypic landscape although no clear patterns emerged.23
There are likely multiple factors that explain the different findings between the present study and that of our European colleagues. Differences in geography, study design, maternal age, access to prenatal care, testing rates, abortion rates, racial/ethnic differences, and survival all likely play a role. Most importantly, it must be recognized that the European studies are population-based analyses with robust case ascertainment. A similar national clinic registry does not exist in the US, and while our database incorporates a plurality of tertiary care pediatric hospitals and likely captures most critical and complex heart disease, it is not comprehensive.
Although we observed a decrease in the proportion of patients with CHD in states with low access to pregnancy termination, this did not reach statistical significance. Prior to the rise of NIPS, there were early concerns that it would lead to increased termination rates in fetuses with DS.24,25 While long-term studies will be necessary to adequately answer this question, there are several smaller studies performed in the US suggesting the opposite: a slight decrease in termination rates between pre- and post-NIPS eras.17 This mirrors the overall pregnancy termination rate trend in the US, which dropped to the lowest recorded rate ever in 2017. Our data support these early studies and suggest that a rise in prenatal detection of DS and coexisting CHD has not significantly influenced parental decisions to terminate pregnancies.
Although no change was seen in proportions of CHD in patients with DS over time across the entire cohort, we also hypothesized that there may be regional differences given state-level variability in access to pregnancy termination. When we stratified the cohort by high vs. low access to termination, we still observed no change over time in the proportion of CHD among patients with DS. However, when looking across the entire timespan of the study, we did find a statistically significant difference in the proportion of AVSD between high-access and low-access states. Whether this difference would persist in larger studies is unknown. Overall, our analyses suggest, as others have hypothesized, that parents use information from prenatal screening and testing to prepare for their child’s birth.
There are several important limitations to our study. Most importantly, our data source only identifies a portion of neonates with DS as described above. While most patients with complex CHD will be captured by the PHIS database due to delivery at or transfer to a PHIS hospital (tertiary care pediatric hospital capable of neonatal congenital heart surgery), there are undoubtedly many patients with balanced AVSDs and VSDs who are never admitted to a tertiary care hospital in the neonatal period. However, it is also true that simple lesions such as VSDs may easily be missed by fetal echocardiography and thus would not play an appreciable role in the decision to terminate a pregnancy. We have no data on prenatal testing rates, termination rates, spontaneous abortion rates, or overall incidence of DS in the population given the lack of a national data registry in the United States. Nonetheless, we feel that our approach uses the best available dataset to help shed light on this important issue. Lastly, access to pregnancy termination has changed over time and our analysis relies upon recent assessments. These limitations prevent us from drawing strong conclusions, but they could inform a more comprehensive population study that includes pregnancy data and a more comprehensive DS cohort.
Conclusions
The proportion of CHD in patients with DS appears to be stable over time despite widespread adoption of NIPS in the US. Small regional variations were observed between states with low vs. high access to pregnancy termination. Population-based studies are needed to completely evaluate the epidemiology of CHD in DS.
References
de Graaf, G., Buckley, F. & Skotko, B. G. Estimates of the live births, natural losses, and elective terminations with Down syndrome in the United States. Am. J. Med. Genet. A 167, 756–767 (2015).
Källén, B., Mastroiacovo, P. & Robert, E. Major congenital malformations in Down syndrome. Am. J. Med. Genet. 65, 160–166 (1996).
Fudge, J. C. et al. Congenital heart surgery outcomes in Down syndrome: analysis of a national clinical database. Pediatrics 126, 315–322 (2010).
Stoll, C., Dott, B., Alembik, Y. & Roth, M.-P. Associated congenital anomalies among cases with Down syndrome. Eur. J. Med. Genet. 58, 674–680 (2015).
Cua, C. L., Haque, U., Santoro, S., Nicholson, L. & Backes, C. H. Differences in mortality characteristics in neonates with Down’s syndrome. J. Perinatol. 37, 427–431 (2017).
Derrington, T. M. et al. Racial/ethnic differences in hospital use and cost among a statewide population of children with down syndrome. Res. Dev. Disabil. 34, 3276–3287 (2013).
Practice Bulletin No. 163: screening for fetal aneuploidy. Obstet. Gynecol. 127, e123–e137 (2016).
Cocchi, G. et al. International trends of Down syndrome 1993-2004: births in relation to maternal age and terminations of pregnancies. Birth Defects Res. A Clin. Mol. Teratol. 88, 474–479 (2010).
Jackson, J. M., Druschel, C. M. & Shapira, S. K. Expanding diagnostic testing beyond cytogenetics: implications for birth defects research and surveillance. Birth Defects Res. A Clin. Mol. Teratol. 97, 726 (2013).
Rink, B. D. & Norton, M. E. Screening for fetal aneuploidy. Semin. Perinatol. 40, 35–43 (2016).
Haymon, L., Simi, E., Moyer, K., Aufox, S. & Ouyang, D. W. Clinical implementation of noninvasive prenatal testing among maternal fetal medicine specialists. Prenat. Diagn. 34, 416–423 (2014).
Greely, H. T. Get ready for the flood of fetal gene screening. Nature 469, 289–291 (2011).
Palomaki, G. E., Knight, G. J., Ashwood, E. R., Best, R. G. & Haddow, J. E. Screening for down syndrome in the United States: results of surveys in 2011 and 2012. Arch. Pathol. Lab. Med. 137, 921–926 (2013).
Norton, M. E. et al. Non-Invasive Chromosomal Evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am. J. Obstet. Gynecol. 207, 137.e1–137.e8 (2012).
Gil, M. M., Quezada, M. S., Revello, R., Akolekar, R. & Nicolaides, K. H. Analysis of cell-free DNA in maternal blood in screening for fetal aneuploidies: updated meta-analysis. Ultrasound Obstet. Gynecol. 45, 249–266 (2015).
Donofrio, M. T. et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation 129, 2183–2242 (2014).
Hill, M. et al. Has noninvasive prenatal testing impacted termination of pregnancy and live birth rates of infants with Down syndrome? Prenat. Diagn. 37, 1281–1290 (2017).
Morris, J. K. et al. Major congenital anomalies in babies born with Down syndrome: a EUROCAT population-based registry study. Am. J. Med. Genet. A 164A, 2979–2986 (2014).
Bergstro, M. S. et al. Trends in congenital heart defects in infants with Down syndrome. Pediatrics 138, e20160123 (2016).
Riehle-Colarusso, T. & Oster, M. E. Down syndrome: changing cardiac phenotype? Pediatrics 138, e20161223 (2016).
Nash, E. Abortion rights in peril—what clinicians need to know. N. Engl. J. Med. 381, 497–499 (2019).
Guttmacher Institute. State abortion policy landscape: from hostile to supportive. https://www.guttmacher.org/article/2019/08/state-abortion-policy-landscape-hostile-supportive (2018).
Pfitzer, C. et al. Dynamics in prevalence of Down syndrome in children with congenital heart disease. Eur. J. Pediatr. 177, 107–115 (2018).
Warsof, S. L., Larion, S. & Abuhamad, A. Z. Overview of the impact of noninvasive prenatal testing on diagnostic procedures. Prenat. Diagn. 35, 972–979 (2015).
Skotko, B. G. With new prenatal testing, will babies with Down syndrome slowly disappear? Arch. Dis. Child. 94, 823–826 (2009).
Author information
Authors and Affiliations
Contributions
S.A.H. conceptualized and designed the study, interpreted the data analysis, drafted the initial manuscript, and reviewed and revised the manuscript. D.N. conceptualized and designed the study, performed the statistical analysis, interpreted the data analysis, and critically reviewed and revised the manuscript for important intellectual content. C.H.B. interpreted the data analysis and critically reviewed and revised the manuscript for important intellectual content. C.L.C. conceptualized and designed the study, interpreted the data analysis, and critically reviewed and revised the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Not required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hart, S.A., Nandi, D., Backes, C.H. et al. Impact of prenatal screening on congenital heart defects in neonates with Down syndrome in the US. Pediatr Res 90, 1081–1085 (2021). https://doi.org/10.1038/s41390-021-01416-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01416-7
This article is cited by
-
A French nationwide study compared various conditions and healthcare use of individuals < 65 years with a Down’s syndrome to those without
Scientific Reports (2023)
-
Initial Counseling Prior to Palliation for Hypoplastic Left Heart Syndrome: 2021 vs 2011
Pediatric Cardiology (2023)
-
Neonatal mortality and morbidity in Down syndrome in the time of prenatal aneuploidy testing: a retrospective cohort study
European Journal of Pediatrics (2022)