Abstract
Background
The aim of this study was to identify factors predicting outcome in patients with mitochondrial disease admitted to pediatric intensive care units (PICU).
Methods
Retrospective study of 2434 patients (age <21 years) admitted to a PICU from 1 January 2006 through 31 March 2016 and captured in the Virtual Pediatric Systems database with ICD9 diagnosis 277.87, disorders of mitochondrial metabolism. Factors influencing mortality and prolonged length of stay (≥14 days) were analyzed using logistic regression.
Results
Predictors independently affecting mortality (adjusted odds ratios and 95% confidence intervals, p < 0.05): age 1–23 months 3.4 (1.7–6.6) and mechanical ventilation 4.7 (2.6–8.6) were risk factors; post-operative 0.2 (0.1–0.6), readmission 0.5 (0.3–0.9), and neurologic reason for admittance 0.3 (0.1–0.9) were factors reducing risk. Predictors affecting prolonged length of stay: mechanical ventilation 7.4 (5.2–10.3) and infectious reason for admittance 2.0 (1.3–3.2) were risk factors, post-operative patients 0.3 (0.2–0.5) had lower risk. The utility of PRISM and PIM2 scores in this patient group was evaluated.
Conclusions
The single most predictive factor for both mortality and prolonged length of stay is the presence of mechanical ventilation. Age 1–23 months is a risk factor for mortality, and infectious reason for admittance indicates risk for prolonged length of stay.
Impact
-
Presence of mechanical ventilation is the factor most strongly associated with negative outcome in patients with mitochondrial disease in pediatric intensive care.
-
Age 1–23 months is a risk factor for mortality, and infectious reason for admittance indicates risk for prolonged length of stay
-
PRISM3 and PIM2 are not as accurate in patients with mitochondrial disease as in a mixed patient population
Similar content being viewed by others
Introduction
Disorders of mitochondrial metabolism are a group of conditions for which the disease course in the intensive care setting is incompletely understood. Prevalence of pathogenic mutations affecting mitochondria is estimated to be 1 in 4300 in the general population.1 Exact prevalence of manifest mitochondrial disease (MD) in children is unknown, but 1 in 11,000–20,000 develop mitochondrial encephalomyopathy during preschool age.2,3 As a group, MDs are among the most commonly inherited neuromuscular disorders.4 Symptoms and clinical signs related to organs with high energy demands are considered common phenotypes of mitochondrial diseases, including cerebral, cardiovascular, and respiratory failure.
In children with MD, early development may be normal before the first episode of metabolic decompensation, often triggered by an episode of increased metabolic demand, such as an infection.5,6 Disease progression is often variable, with recurrent clinical deterioration and recovery.7,8,9 Patients with manifest MD have an average of 16 symptoms,10 mostly from organs with high energy requirements such as skeletal muscle, heart,11 and nervous system.12
Metabolic decompensation necessitating PICU admission may be directly related to the patient’s MD or precipitated by an acute illness, such as a viral infection. The clinical characteristics of patients with MD admitted to the pediatric intensive care unit (PICU) has not been systematically studied. It is also unknown what factors affect mortality and prolong length of stay, and how currently used predictive scores perform in this unique patient population.
The purpose of the study was therefore to describe the clinical characteristics and factors that are associated with mortality and PICU length of stay in patients diagnosed with disorders of mitochondrial metabolism admitted to the PICU. We also investigated how well the severity of illness scores Pediatric Index of Mortality 2 (PIM2),13 a second-generation mortality prediction tool in PICUs, and Pediatric Risk of Mortality (PRISM) 3, a third-generation pediatric physiology-based score for mortality risk, performed in these patients.14
Materials and methods
Study design and selection of subjects
This was a retrospective descriptive study using the Virtual Pediatric Systems (VPS) database combining data on PICU admissions from 130 different hospitals primarily in North America. Study subjects included all patients who were captured in the VPS database from 1 January 2009 through 31 March 2016 with the STAR/ICD9 code 277.87 (disorders of mitochondrial metabolism), here referred to as mitochondrial disease, (MD) up to 21 years of age at time of admission. The STAR/ICD9 code 277.87 includes mitochondrial diseases such as MELAS (mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes), MERFF (myoclonic epilepsy with ragged-red fibers), MNGIE (mitochondrial neurogastrointestinal encephalopathy disease), NARP (neuropathia ataxia retinitis pigmentosa), and Kearns–Sayre syndrome, but is not exhaustive for all kind of mitochondrial disease (e.g. Leigh syndrome is usually not coded using 277.87). The study was conducted in full accordance of all applicable Children’s Hospital of Philadelphia Research Policies and Procedures and all applicable federal and state laws and regulations including 45 CFR 46, and the HIPAA Privacy Rule. Data collected included the majority of the available data in the VPS database for the requested patient population. Aggregated descriptive data (not individual data points) for patients with other diagnoses than MD from the same time period were also collected from the VPS.
Study endpoints
Data for MD patients admitted for PICU care were grouped based on survival and presented descriptively. The outcomes for regression analyses were PICU mortality and prolonged length of PICU stay (here defined as ≥14 days). For PRISM and PIM2 scores, area under the curve (AUC) of receiver operating characteristic (ROC) curves for prediction of mortality were created and analyzed.
Statistical analysis
Data were analyzed and figures created using Stata 16.0 (StataCorp, College Station, TX). Categorical data are presented as count (percent) while continuous variables are presented as median (interquartile range, IQR). Statistical analysis was performed using logistic regressions models individually for the two study endpoints. First, a univariate logistic regression analysis of each predictor was performed. Then a fully adjusted multivariate logistic regression (each predictor is fully adjusted for all the other predictors) was performed with all cases with a full data set. Odds ratios with 95% confidence intervals (95% CI) were calculated for each parameter for each endpoint, first using in the univariate model, and then in the fully adjusted model.
The performance of PRISM and PIM2 scores were assessed by area under the ROC curve for discrimination and Hosmer–Lemeshow goodness of fit test p values. A p value of 0.05 was considered statistically significant.
To compare performance between centers handling many cases of PICU admitted MD patients with centers handling fewer, centers were dichotomized into larger (≥100 cases during the period) and smaller centers (<100 cases). Hosmer–Lemeshow test for goodness of fit across deciles at risk was performed, and standard mortality ratios (SMR) were calculated for each group based on the probability of death predicted by PRISM3 as coded in VPS.
Results
Demographics
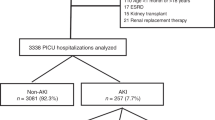
Basic clinical characteristics for 2434 individual cases of PICU admittance with ICD9 code 277.87 (disorders of mitochondrial metabolism) between 1 January 2009 through 31 March 2016, and an aggregate of PICU admittances for all other diagnoses during the same time period were summarized (Table 1). The overall mortality in the MD group was 5.2% compared to 2.4% in the aggregate group. Descriptive statistics of the cohort of MD patients depending on PICU mortality is presented in Table 2. The most prevalent main reason for PICU admittance was respiratory, with 634 cases (26%), followed by neurologic (457 cases, 19%), infectious (315 cases, 13%), and metabolic (299 cases, 12%).
PICU mortality
The cohort was analyzed for factors affecting PICU mortality. All 2434 cases of PICU admittance of MD patients were initially analyzed using a univariate logistic regression analysis of each predictor of PICU mortality (Table 3, column A). In total, 2061 cases had a full data set and could be included in the multivariate logistic regression (where each predictor is fully adjusted for all the other predictors) (Table 3, column B). In the unadjusted model, neonates, patients aged 1–23 months, and patients aged above 18 had a significantly increased odds ratio (OR) of PICU mortality while patients in the span of 2–12 years appeared more likely to survive. In the adjusted model, only patients aged 1–23 months had a significantly increased OR. No race effect could be seen in the adjusted model, even though Hispanics appeared to have increased risk of mortality in the unadjusted model. Patients who were readmitted fared better compared to first-time patients in either model and so did post-operative patients, both groups with low ORs. Presence of invasive ventilation was the strongest independent risk factor for PICU death both in the unadjusted and the adjusted models. In the unadjusted model, cardiovascular and metabolic reasons for admittance were related to worse outcomes, but these effects did not persist after adjusting for the other clinical parameters. In the adjusted model, neurologic reason for admission was associated with a lower risk of death (the majority of these patients were admitted due to seizures), while no organ class significantly increased risk compared to the reference. Both trauma and prolonged length of stay resulted in higher OR for mortality in the unadjusted model, but these effects were no longer apparent after adjustment. The OR for each increasing unit of PRISM score was 1.2 in both models. A fully adjusted analysis where post-operative patients and trauma patients were excluded was also performed, with no relevant changes in the results (1676 cases analyzed).
Prolonged length of PICU stay
A similar analysis utilizing a univariate logistic regression followed by a fully adjusted multivariate logistic regression for the outcome “prolonged length of stay” (here defined as a length of stay in the PICU for 14 days or longer) was performed (Table 4). Neonates and patients 1–23 months of age had an increased risk of prolonged stay in the unadjusted analysis, with the effect still being significant in the fully adjusted model for children aged 1–23 months. Readmittance decreased the risk, and so did post-operative care. The presence of invasive mechanical ventilation markedly increased the risk of prolonged PICU hospitalization, the strongest effect seen in the adjusted model. One organ class stood out; in the fully adjusted model infectious reason for admittance doubled the risk of prolonged PICU stay. An additional adjusted analysis for prolonged length of stay excluding trauma patients and post-operative patients (1676 cases analyzed) was performed, and here the OR for the 1–23 months age group was minimally attenuated but directionally consistent, 1.6 (95% CI 1.0–2.5) and it just exceeded statistical significance (p = 0.051).
PRISM and PIM2
PIM2 scores and PRISM score were analyzed using ROC curves to test the ability of these scores to predict mortality in this particular patient group (Fig. 1). The AUC (95% CI) ROC for PIM2 was 0.78 (0.73–0.82), and for PRISM 0.77 (0.72–0.82). The distribution of PIM2 scores and PRISM scores, respectively, in patients alive at PICU discharge and deceased at discharge is displayed in Fig. 2. Exclusion of post-operative and trauma patients did not affect the AUC for either score.
We also performed Hosmer–Lemeshow (HL) test for goodness of fit and calculated SMR for MD patients based on PRISM predicted mortality for large centers (≥100 observations during the inclusion period) versus smaller centers (<100 observations). The SMR for the former was 1.00 (95% CI 0.64–1.46) and for the latter 1.00 (95% CI 0.81–1.21). Due to too few cases, the confidence intervals are wide, and no conclusions could be made regarding if stratifications of these patients to more specialized centers benefit this patient groups or not. The p values for HL was 0.199 and <0.001, respectively, the latter indicating inadequate fitting to the model, likely also primarily a result of too few occurrences of death for this analysis. The SMR for the whole cohort was 1.00 (0.83–1.19) and the HL p value <0.001.
Discussion
We analyzed data from more than 2000 cases of PICU hospitalizations of MD patients from primarily North America during the period 1 January 2009 through 31 March 2016. Presence of invasive mechanical ventilation and age 1–23 months were predictors of higher mortality. Patients readmitted to a PICU had a significantly lowered OR for death, possibly reflecting the waxing and waning disease course of many MDs, with metabolic crisis at times of increased metabolic demand, but also a natural recovery as the factor causing the crisis cedes, such as in case of temporally self-limiting infection. Patients with neurologic reasons for PICU admittance had a reduced OR for death and the most common reason for admittance for these patients was seizures. The presence of invasive mechanical ventilation, and age 1–23 months also increased the likelihood of a prolonged length of PICU stay, and so did admittance due to infectious reasons, reflecting the sensitivity to being burdened with the additional metabolic strain connected to an infection in these patients. Patients admitted to a PICU post-operatively not surprisingly fared better both regarding mortality and length of stay.
We have also shown that standard indices of disease severity to some extent serve to predict outcome in these patients, although the 0.773 ROC AUC for PRISM3 scores and the 0.775 ROC AUC for PIM2 scores in this material is lower than what has been described for either scores for a broader group of patients (about 0.90 for both).14,15 This discrepancy indicates that these standard indices are not optimized for the MD patient group, and that the predictive ability of these scores is not as good as in a mixed pediatric patient population. The results do not provide any guidance as to whether either is better at predicting outcome in this patient population.
Patients with MD are a complex patient group with heterogeneous presentation, disease course, and prognosis. Symptomatology and morbidity from MD may span from mild clinical symptoms barely affecting daily life to devastating multi-organ dysfunction at an early age. The symptomatology is likely to vary with function of the mitochondria and bioenergetic needs at baseline and during stress, with the extreme end being total mitochondrial dysfunction which is incompatible with human life. Cellular ATP requirement is dynamic and vary with the activity of the cell, tissues, organs, and ultimately individual. Physical strain such as exercise or illness can affect disease course and cause a metabolic crisis leading to clinical disease. Alternatively, reduced substrate supply for mitochondrial metabolism can hamper an already partly dysfunctional ATP production and lead to worsened disease, e.g. in cases of fasting. Thus, in patients with MD even a relatively commonplace affliction, such as an upper airway infection, potentially can lead to severe disease and PICU hospitalization.
Organ systems most commonly affected by MD are those with high energy requirements, such as the central nervous system and the heart. In this study, the organ system most commonly reported as the primary reason for PICU admittance is respiratory failure, but the database does not delineate whether this is the primary disease-causing event or a secondary feature. These data do not single out one organ class that have a higher risk of mortality, but demonstrate that patients with infections have a higher risk of prolonged PICU stay. Due to the mechanisms outlined above, patients with MD are more severely affected by infections due to the secondary cascade of energetic failure the increased strain on the system cause. Neurologic reasons for admittance came with a reduced risk of death, but not with a reduced risk of prolonged length of stay, possibly reflecting that seizures in themselves seldom are immediately lethal but may result in prolonged hospitalization.
In recent years, centers that specialize in patients with MD have been established, and in our cohort for example, the top 10 hospitals, out of over 130 account for nearly half (45.6%) of all admissions. It could be speculated that centralizing care for these patients would improve quality, but the analyses performed in this study could neither prove nor disprove this. There are concerns of variations between centers in the care and treatment of these patients, which is not surprising considering the lack of evidence-based treatment options.16 The Mitochondrial Medicine Society has published a consensus statement with recommendations on the treatment of these patients17 including a section on critical care with some concrete recommendations such as that the use of valproate for seizure control must be avoided in these patients as fulminant hepatic failure has been associated with this medication,17 but most of the recommendations are of generic nature and applicable to all critically ill patients. Although there naturally are similarities, such as worse outcome in patients on mechanical ventilation, our findings demonstrate that children with MD not necessarily have the same risk factors for adverse outcomes than other patients admitted to the PICU,18 thus there is a clear knowledge gap that needs to be addressed.
While this study provides insights regarding a large number of MD patients admitted to the PICU, the study has clear limitations. Parameters available for analysis were restricted to those recorded in VPS, and did not include blood lactate, a clinical parameter traditionally considered of high relevance for patients with mitochondrial disease (base excess is reported as a part of the PIM2 score in VPS, but was only available in 11,6% of reported cases, and hence was not further analyzed). The data set is limited by the STAR/ICD9 code 277.87 which includes MELAS, MERFF, MNGIE, NARP, and Kearns–Sayre syndrome; however, certain other diagnoses may not be included. A notable example is Leigh syndrome, where patients do not routinely get the diagnosis code 277.87, but rather the non-specific ICD9 code 330.8 (“other specified cerebral degenerations of childhood”) even though it is undoubtedly an MD.19 This study is therefore not encompassing the full width of the MD spectrum. Also, the VPS database includes patients up to 21 years of age, an age that cannot be considered pediatric. The cases aged above 18 years make up 2.2% of the cohort. The current study used ICD9 codes; the ICD10 system include codes that more precisely codes for various MDs and would allow for both better coverage of the full MD field and also better resolution. This is important both due to the increasing accuracy for diagnostics of mitochondrial disorders and evolving diagnostic criteria20 and future studies may provide further granularity. There is also a need to make a thorough analysis to define how this particular group of patients differ from other groups of PICU patients, and how to best optimize their care and treatments.
Conclusions
We conclude that among children with MD admitted to a PICU, patients aged 1–23 months both have a higher likelihood of death and a higher likelihood of prolonged stay. Readmitted patients fare better. Infectious reason for admittance increases the likelihood of a prolonged stay, while neurologic reason for admittance reduce the risk of death. The single strongest predictor for both death and prolonged stay was the presence of mechanical invasive ventilation. How to best optimize intensive care for these patients is not yet well defined, and we encourage increased vigilance by clinicians caring for the critically ill MD patient.
References
Gorman, G. S. et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann. Neurol. 77, 753–759 (2015).
Darin, N., Oldfors, A., Moslemi, A. R., Holme, E. & Tulinius, M. The incidence of mitochondrial encephalomyopathies in childhood: clinical features and morphological, biochemical, and DNA anbormalities. Ann. Neurol. 49, 377–383 (2001).
Skladal, D., Halliday, J. & Thorburn, D. R. Minimum birth prevalence of mitochondrial respiratory chain disorders in children. Brain 126, 1905–1912 (2003).
Schaefer, A. M. et al. Prevalence of mitochondrial DNA disease in adults. Ann. Neurol. 63, 35–39 (2008).
Chinnery, P. F. & Turnbull, D. M. Clinical features, investigation, and management of patients with defects of mitochondrial DNA. J. Neurol. Neurosurg. Psychiatry 63, 559–563 (1997).
Dixon, M. A. & Leonard, J. V. Intercurrent illness in inborn errors of intermediary metabolism. Arch. Dis. Child. 67, 1387–1391 (1992).
Kerr, D. S. Review of clinical trials for mitochondrial disorders: 1997-2012. Neurotherapeutics 10, 307–319 (2013).
Sofou, K. et al. A multicenter study on Leigh syndrome: disease course and predictors of survival. Orphanet J. Rare Dis. 9, 52 (2014).
DiMauro, S. & Schon, E. A. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 348, 2656–2668 (2003).
Zolkipli-Cunningham, Z. et al. Mitochondrial disease patient motivations and barriers to participate in clinical trials. PLoS ONE 13, e0197513 (2018).
Bates, M. G. et al. Cardiac involvement in mitochondrial DNA disease: clinical spectrum, diagnosis, and management. Eur. Heart J. 33, 3023–3033 (2012).
DiMauro, S. Mitochondrial diseases. Biochim. Biophys. Acta 1658, 80–88 (2004).
Pollack, M. M. et al. The Pediatric Risk of Mortality Score: update 2015. Pediatr. Crit. Care Med. 17, 2–9 (2016).
Pollack, M. M., Patel, K. M. & Ruttimann, U. E. PRISM III: an updated Pediatric Risk of Mortality score. Crit. Care Med. 24, 743–752 (1996).
Slater, A., Shann, F. & Pearson, G., Paediatric Index of Mortality Study, G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 29, 278–285 (2003).
Pfeffer, G., Majamaa, K., Turnbull, D. M., Thorburn, D. & Chinnery, P. F. Treatment for mitochondrial disorders. Cochrane Database Syst. Rev. 4, CD004426 (2012).
Parikh, S., et al. Patient care standards for primary mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genet. Med. 19 https://doi.org/10.1038/gim.2017.107 (2017).
Heneghan, J. A. et al. Characteristics and outcomes of critical illness in children with feeding and respiratory technology dependence. Pediatr. Crit. Care Med. 20, 417–425 (2019).
Gerards, M., Sallevelt, S. C. & Smeets, H. J. Leigh syndrome: resolving the clinical and genetic heterogeneity paves the way for treatment options. Mol. Genet. Metab. 117, 300–312 (2016).
Parikh, S. et al. Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genet. Med. 17, 689–701 (2015).
Acknowledgements
We thank the staff of the Children’s Hospital of Philadelphia (CHOP) Critical Care Center for Evidence and Outcomes for their efforts in abstracting and coding the CHOP Virtual Pediatric Systems (VPS) data used to prepare this report. VPS data were provided by VPS, LLC. No endorsement or editorial restriction of the interpretation of these data or opinions of the authors has been implied or stated. This work is funded through institutional funds at The Children’s Hospital of Philadelphia (Kilbaugh) and at Lund University (Ehinger).
Author information
Authors and Affiliations
Contributions
M.K. and T.J.K. conceived the study. M.K., T.J.K., S.E.M., S.E.K., and M.J.F. wrote the study plan. S.E.K. collected and organized data. J.K.E., M.K., T.J.K., F.S., and M.L. analyzed the data. J.K.E. and A.Å. planned and performed the statistical analyzes. J.K.E. wrote the manuscript with M.K. and T.J.K. All authors critically reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
This work was exempt from patient consent by the IRB of The Children’s Hospital of Philadelphia.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ehinger, J.K., Karlsson, M., Sjövall, F. et al. Predictors of outcome in children with disorders of mitochondrial metabolism in the pediatric intensive care unit. Pediatr Res 90, 1221–1227 (2021). https://doi.org/10.1038/s41390-021-01410-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01410-z