Abstract
This study reports on the human milk fortification session at the 2019 NEC Society Symposium, which included clinicians and parents discussing the evidence comparing fortification options such as efficacy, safety, cost effectiveness, and the need for parents to be informed about fortifier choice. With the current literature available and the varying standard of care practices for human milk fortification, further studies are needed to determine the most complete diet for preterm infants. The optimal diet would not only provide key nutrients and energy for growth and development, but also improve short- and long-term outcomes. Parents, as advocates and providers for their infant, should be informed, educated, and included in the discussion and decisions regarding fortification of human milk for their infant.
Similar content being viewed by others
Introduction
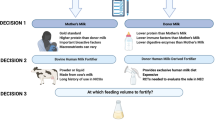
Mother’s milk is the ideal source of nutrition for infants; however, preterm infants have increased nutritional and energy needs during a critical stage of growth and development outside the womb. The preterm infant often requires higher nutrient intake than either the fetus or the full-term newborn due to the preterm infant’s need to simultaneously complete fetal development and to assume the metabolic demands of a newborn. Preterm infants have limitations in feeding volume due to comorbidities and, when orally feeding, have compromised self-regulation of nutrient intake. Therefore, to provide adequate nutrition to a preterm infant, nutrient supplementation beyond mother’s milk is required.
Higher nutrient delivery to preterm infants is associated with improved growth, and improved growth during birth hospitalization is associated with less neurodevelopmental delay.1,2,3,4,5 Identifying the method to promote optimal nutrient delivery in a safe manner is critical to preterm infant outcomes. Nutrition can be delivered parenterally by an intravenous route, but this is not the physiologic way to feed infants and can be associated with toxicities and with central line-associated blood stream infections. Thus, the safest method for nutrient delivery to preterm infants appears to be through enteral feeding. The addition of nutrients may be provided by supplementation with individual additives such as carbohydrate, fat, protein, calcium, and phosphorus or supplementation with concentrated infant formula or a multi-nutrient human milk fortifier. Multi-nutrient human milk fortifiers are often concentrated infant formula and, therefore, are bovine milk-based fortifiers (BMBFs). A multi-nutrient human milk fortifier derived from donated, pasteurized human milk [human milk-based fortifier (HMBF)] is available at some institutions mainly in the United States and Europe (Prolact + H2MF® by Prolacta Bioscience, Industry, CA). Identifying whether these fortifiers can safely deliver added nutrition is critical to preterm health.
The preterm infant intestine is a fragile environment with a thin gastrointestinal mucous layer, increased intestinal permeability, and a dysbiotic microbiome, making it more susceptible to necrotizing enterocolitis (NEC)—the most severe consequence of intestinal immaturity. Intake of mother’s milk is associated with improvement in intestinal function, including decreased intestinal permeability and less oxidative stress compared to preterm infant formula feeding.6,7 The addition of BMBF has also been shown to alter the innate immune-functioning properties of human milk and increases preterm infant gastrointestinal oxidative stress.7,8 Furthermore, digestion and absorption of the nutrition provided from human milk fortification is a concern. Delivery of increased nutritional density will not benefit the infant if the gastrointestinal mechanisms to digest, absorb, and utilize the nutrients are not functional. In fact, undigested components may interact with colonic bacteria in a manner detrimental to the infant. This review provides an overview of the evidence regarding critical questions in the comparison of fortification options, such as efficacy, safety, cost effectiveness, and the need for parents to be informed and included in discussion about fortification.
Human milk and especially mother’s milk for the very low birth weight infant
Very low birth weight infants experience less late-onset sepsis, NEC, and mortality when human milk is fed instead of preterm infant formula.9,10,11 Specifically, for NEC, early studies showed a significant decrease in occurrence with mother’s milk at doses of 50% of total enteral feeds and 50 ml/kg/day.9,10,12 A large study in extremely low birth weight infants showed that every 10% incremental increase in human milk intake in the first 2 postnatal weeks was associated with a significant decreased risk of NEC or death.11 Although the evidence regarding the benefit of donor human milk is less pronounced than for mother’s milk, a meta-analysis concluded significantly less risk of NEC with intake of donor human milk compared to preterm formula; however, formula-fed infants had better short-term growth.13 There was no difference in all-cause mortality, long-term growth, or neurodevelopment.13 Due to the significant decrease in the incidence of NEC with the use of donor human milk, mother’s milk supplemented with donor human milk is the most beneficial and safest method to feed preterm infants in the neonatal intensive care unit (NICU).14,15
Parents commonly are informed of the benefit and safety of mother’s milk and donor human milk feeds.16,17 They are informed of the benefit of mother’s milk to encourage mothers to initiate and sustain milk expression during her preterm infant’s hospitalization. They are educated about the benefits and safety of feeding pasteurized donor human milk instead of preterm infant formula because most institutions require parental consent or assent for donor human milk. However, despite these common discussions regarding preterm infant feeding, parents may not receive information on the nutritional deficits that occur with human milk feeding to preterm infants or the benefits and risk of human milk fortification.
Fortification of human milk for preterm infants
An important question asked by parents is—if milk is so medicinal, why do we risk compromising its and my infant’s safety by adding fortifier? Before the advent of human milk fortifier, preterm infants receiving mother’s milk exhibited slower growth and greater metabolic dyscrasias when compared to formula-fed infants18,19 Therefore, the standard recommendations are to support fortified mother’s milk feeding for preterm infants.14,20
The nutrients thought to be the most deficient for a preterm infant-fed human milk alone are calcium, phosphorus, protein, and energy. Eighty percent of the calcium and phosphorus accrued by a fetus occurs in the third trimester, with peak accrual at 36–38 weeks’ postmenstrual age (PMA). Table 1 demonstrates the recommended intake and concentrations provided by human milk and fortified human milk of these minerals, energy, and protein.
Multicomponent fortifier became the mainstay of in-hospital preterm infant nutrition in the 1990s. These fortifiers included not only calcium, phosphorus, protein (from bovine, cow’s milk source), and energy (commonly as medium chain triglycerides, vegetable oil, and corn syrup solids), but also other minerals, vitamins, trace elements, and polyunsaturated fatty acids. Overall, meta-analysis of studies of these multicomponent fortifiers has concluded that they are associated with greater weight, length, and head circumference gain, and they have no significant association with higher neurodevelopmental scores or risk for NEC (Table 2).21 Although the current evidence does not demonstrate an increased risk of NEC with exposure to BMBF, with the association between preterm infant formula and NEC, the concern persists that exposure to a bovine product may have consequences that have yet to be adequately studied. Through preterm infant family support groups and social media, parents express that they are exposed to descriptions of infants who experienced a temporal association of milk fortification with a multicomponent fortifier and the occurrence of NEC. This parent perspective was voiced at the 2019 NEC Society Symposium session on human milk fortification. These concerns raised a critical question—to what degree should parents be informed of the risk/benefits of human milk fortification?
HMBF compared to BMBF
Although concentrated human milk protein and fat supplements were given to preterm infants in the 1980s, it was not until 2009 that a HMBF made from pasteurized donor human milk became commercially available (Prolact + H2MF®).22 Hence, an exclusive human milk-based diet (EHM diet), which included mother’s own milk supplemented with donor human milk and fortification with HMBF, was available. In a randomized controlled trial (RCT) published in 2010, infants who received an EHM diet compared to a mixed bovine diet (mother’s own milk fortified with bovine fortifier and/or formula) showed a 60% reduction in NEC and a 90% reduction in surgical NEC.23 Another arm of the study compared EHM diet to only preterm formula and showed a decrease in both NEC and parenteral nutrition days.24 Furthermore, a multicenter observational study of over 1500 infants comparing a pre-intervention group receiving a mixed bovine diet compared to the post-intervention group receiving an EHM diet showed decreased NEC, mortality, late-onset sepsis, bronchopulmonary dysplasia, and retinopathy of prematurity in infants fed all human milk.25 Of note, in all of these studies, EHM diet was compared to a diet that contained not only BHMF, but also preterm formula. In 2007 when the Sullivan study was being conducted, there was limited use of supplemental donor human milk and many infants received preterm formula if mother’s own milk was not available. Now, in 2019, donor human milk is widely used by NICUs in the United States. Due to this change in nutrition practice, it is unclear if in the setting of a base diet consisting of mother’s own milk supplemented with donor human milk fortified with HMBF vs. BHMF impacts outcomes in premature infants.
A recent triple-blinded RCT by O’Connor et al.26 compared a HMBF to a powdered BMBF with no confounding preterm formula and found no difference in the primary outcome of feeding intolerance between the two groups. The study was small in sample size and showed no difference in NEC. In the study, the HMBF group had a lower incidence of severe retinopathy of prematurity.
Currently, there is an ongoing randomized controlled multicenter trial in Sweden (Swedish Trial, NCT03797157) comparing a HMBF to a BMBF in extremely preterm infants <28 weeks gestation.27 Infants in the study will receive mother’s own milk supplemented with donor human milk and then will be randomized to either HMBF or BMBF and will be evaluated for the composite score of NEC, culture-proven sepsis, and mortality.27 This fortifier study aims to answer the question if mother’s milk is supplemented with donor human milk and formula is avoided, does the type of fortifier (human vs. bovine) alter outcomes?
In addition to the study of clinical benefit, due to the high cost of HMBF, two retrospective studies have investigated the cost effectiveness of HMBF and demonstrated a cost reduction with the use of EHM diet (no preterm formula or BHMF) associated with a decrease in NEC cases.28,29 However, the cost of HMBF is a common barrier in the NICU because the budget for a HMBF exceeds what is allotted for nutrition at most institutions. While some payer sources in the United States (U.S.) reimburse for the cost of the HMBF, reimbursement is not consistent.
In a recent survey by Parker et al.30 of NICU medical directors (44% responded), 88% of Level 3 and 4 NICUs reported routine donor human milk use. Of the surveyed NICUs, 44% used HMBF. With the increased use of HMBF and donor human milk in the U.S. and the new availability of HMBF in Europe, clinicians should be prepared to respond to parental questions about variation in feeding practices and the availability of bovine and HMBFs.
Discussion with parents regarding human milk fortification
Infants receiving human milk and BMBF experience less NEC than infants fed preterm formula.9,10,12 Although the combined cost of BMBF with programs to support mother’s milk production and donor human milk is higher than the cost of preterm infant formula, the evidence of benefit appears to outweigh the cost. With the known risks of formula to the preterm infant’s intestinal tract, although no studies comparing BMBF directly with HMBF have shown harm, does the potential benefit of fortification with HMBF outweigh the cost? That is a question that remains to be answered. Nonetheless, other nutrient interventions have been adopted as standard of care with less research support. The benefit of HMBF is being held to a high standard because it must outweigh the expense of the product, but current trials have been small, and most have compared an EHM to a mixed bovine diet. Table 3 lists some of the barriers to implementing an exclusive human milk diet. For parents, it may be upsetting and confusing when hearing that cost is one of the calculated components when deciding whether or not to use HMBF. It is important to recognize that some clinicians, institutions, and parents will decide that the potential benefit of HMBF outweighs the cost. The ongoing Swedish Study may be able to answer the head-to-head comparison of bovine and HMBFs in the absence of formula use; however, the primary outcome (a composite score of NEC, sepsis, and mortality) may not provide conclusive data about NEC.27
Another consideration regarding the choice of fortification that is important to clinical care providers and to parents is the displacement of mother’s milk by fortifier. As mentioned previously, the studies regarding mother’s milk protection against NEC demonstrate a dose response. Some institutions use powdered fortification instead of liquid fortification to decrease displacement and maximize the dose of mother’s milk to the volume-restricted preterm infant. Liquid fortifiers are popular in the U.S. because the liquid fortifiers provide sterility unlike in powdered formula, which cannot be sterilized. Growth of Cronobacter sakazakii in powdered formula raised this concern, although no infant is known to have contracted C. sakazakii through bovine human milk fortifier.31
Should the clinician’s rationale regarding human milk fortification choices be shared with parents prior to fortification of the infant’s feeds? Based on the question and answer session following the human milk fortification lecture at the 2019 NEC Society Meeting, parents voiced that it is reasonable for parents to expect to receive clear, helpful information on the topic from their infant’s clinical care team. Parents felt that the clinical care team should provide a thoughtful, evidence-based approach to human milk fortification decisions and share this approach with parents. If the approach includes an opportunity for a choice for fortification, parents would like to be included in the decision-making process. The best example of this is for discharge nutrition where multiple options exist with minimal evidence regarding the correct choice for a given family.
For fortification with HMBF, parental choice may be less available. A hospital may decide to have the HMBF available to all preterm infants, a subset of high-risk infants, have an option for parents to purchase the fortifier independently, or not have the HMBF as an option. No matter the choice, parents have expressed that they can learn from their own research that practices vary between units and are entitled to question and be informed of the institution’s choice. Parents feel that if clinicians are having conversations with parents regarding mother’s milk and donor human milk, information regarding the institution’s fortification practices should be added to these discussions. Parental awareness of the intent and expectations with fortification is a preemptive step to promote open and honest discussion between caregivers and parents, especially if an infant develops NEC. In addition, former NICU parents have expressed that they would like to be involved at a higher level of decision making, such as a parent advocate on the leadership committee for the NICU. This is another opportunity for clinician and parent discussion and shared decision making.
In these discussions, clinical care providers should consider how medical terminology is communicated. First, the term “human milk fortifier” may be interpreted as being made from human milk instead of its intent to describe a supplement to human milk. Parents describe that they are not given the details necessary to recognize that a bovine or cow’s milk-based product was received by their preterm infant. Therefore, a revision of labels to “bovine milk-derived fortifier for human milk” or “human milk-derived fortifier for human milk” would provide clarification and avoid the circumstance where parents feel misled by the term. Second, parents want to know about fortification—the benefits and potential risks. Therefore, care providers have the responsibility to know and describe what they are giving an infant, why it is being given, the potential risks with its use, and what to expect.
Conclusion
The optimal nutrition for premature infants not only provides key nutrients and energy for growth and development, but also improves short- and long-term outcomes. HMBF is a product available in some institutions as a method to provide an EHM diet (mother’s milk, donor human milk, and HMBF); however, barriers exist to implementation in many NICUs and questions remain as to the extent of benefit of HMBF over BMBF. An EHM diet is associated with decreased morbidity and mortality when compared to infants receiving BMBF and formula. There is insufficient data to determine the beneficial effects of HMBF against BMBF as only one small blinded, RCT tested this with use of mother’s own milk and donor human milk.
With the growing amount of information on the internet and social media, many questions arise regarding different nutrition practices. Parents, as advocates and providers for their infant, should be informed and included in the discussion and decisions regarding fortification of human milk for their infant. No matter the choice or circumstance, information about fortifiers should be shared with parents as they navigate their infant’s NICU course.
Future research directions
Key questions regarding the use of HMBF vs. BMBF still remain and should be priorities for research in the field of neonatal nutrition. While DHM has been shown to decrease the incidence of NEC in preterm infants, long-term benefits are unclear. Further research should focus on evaluating long-term benefits of DHM. An adequately powered high-quality trial is needed in order to determine the benefits of using HMBF compared to BMBF in the setting of mother’s milk supplemented with donor human milk without the use of formula. While a 222 preterm infant RCT fortifier trial is currently being conducted in Sweden, larger RCTs may be needed; however, this may be a challenge due to the cost of the HMBF for study use and the limitation on the numbers of centers that could participate in the study due to increasing use of HMBF in U.S. NICUs. Lastly, further research should focus on the pathophysiology of the preterm infant intestine exposed to HMBF or BMBF.
References
Shah, P. S. et al. Postnatal growth failure in preterm infants: ascertainment and relation to long-term outcome. J. Perinat. Med. 34, 484–489 (2006).
Ehrenkranz, R. A. et al. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 117, 1253–1261 (2006).
Belfort, M. B. et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics 128, e899–e906 (2011).
Zozaya, C., Diaz, C. & Saenz de Pipaon, M. How should we define postnatal growth restriction in preterm infants? Neonatology 114, 177–180 (2018).
Franz, A. R. et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics 123, e101–e109 (2009).
Taylor, S. N., Basile, L. A., Ebeling, M. & Wagner, C. L. Intestinal permeability in preterm infants by feeding type: mother’s milk versus formula. Breastfeeding medicine: the official journal of the Academy of Breastfeeding. Medicine 4, 11–15 (2009).
Friel, J. K. et al. Evidence of oxidative stress in relation to feeding type during early life in premature infants. Pediatr. Res. 69, 160–164 (2011).
Jocson, M. A., Mason, E. O. & Schanler, R. J. The effects of nutrient fortification and varying storage conditions on host defense properties of human milk. Pediatrics 100(Part 1), 240–243 (1997).
Schanler, R. J., Shulman, R. J. & Lau, C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 103(Part 1), 1150–1157 (1999).
Furman, L., Taylor, G., Minich, N. & Hack, M. The effect of maternal milk on neonatal morbidity of very low-birth-weight infants. Arch. Pediatr. Adolesc. Med. 157, 66–71 (2003).
Meinzen-Derr, J. et al. Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J. Perinatol. 29, 57–62 (2009).
Sisk, P. M., Lovelady, C. A., Dillard, R. G., Gruber, K. J. & O’Shea, T. M. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J. Perinatol. 27, 428–433 (2007).
Quigley, M., Embleton, N. D. & McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 6, CD002971 (2018).
Arthur I. E. et al. Breastfeeding and the use of human milk. Pediatrics 129, e827–e841 (2012).
Arslanoglu, S. et al. Donor human milk for preterm infants: current evidence and research directions. J. Pediatr. Gastroenterol. Nutr. 57, 535–542 (2013).
Fleurant, E. et al. Barriers to human milk feeding at discharge of very-low-birth-weight infants: maternal goal setting as a key social factor. Breastfeed. Med. 12, 20–27 (2017).
Meier, P. P., Johnson, T. J., Patel, A. L. & Rossman, B. Evidence-based methods that promote human milk feeding of preterm infants: an expert review. Clin. Perinatol. 44, 1–22 (2017).
O’Connor, D. L. et al. Growth and development of premature infants fed predominantly human milk, predominantly premature infant formula, or a combination of human milk and premature formula. J. Pediatr. Gastroenterol. Nutr. 37, 437–446 (2003).
Alpay, F. et al. Measurement of bone mineral density by dual energy X-ray absorptiometry in preterm infants fed human milk or formula. Eur. J. Pediatr. 157, 505–507 (1998).
Agostoni, C. et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 50, 85–91 (2010).
Brown, J. V., Embleton, N. D., Harding, J. E. & McGuire, W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database Syst. Rev. 5, Cd000343 (2016).
Polberger, S. K., Axelsson, I. A. & Raiha, N. C. Growth of very low birth weight infants on varying amounts of human milk protein. Pediatr. Res. 25, 414–419 (1989).
Sullivan, S. et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J. Pediatr. 156, 562–567.e561 (2010).
Cristofalo, E. A. et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J. Pediatr. 163, 1592–1595 e1591 (2013).
Hair, A. B. et al. Beyond necrotizing enterocolitis prevention: improving outcomes with an exclusive human milk-based diet. Breastfeed. Med. 11, 70–74 (2016).
O’Connor, D. L. et al. Nutrient enrichment of human milk with human and bovine milk-based fortifiers for infants born weighing <1250 g: a randomized clinical trial. Am. J. Clin. Nutr. 108, 108–116 (2018).
Abrahamsson, T. R. et al. Human milk fortification in extremely preterm infants (Nordic Trial). https://clinicaltrials.gov/ct2/show/NCT03797157.
Assad, M., Elliott, M. J. & Abraham, J. H. Decreased cost and improved feeding tolerance in VLBW infants fed an exclusive human milk diet. J. Perinatol. 36, 216–220 (2016).
Ganapathy, V., Hay, J. W. & Kim, J. H. Costs of necrotizing enterocolitis and cost-effectiveness of exclusively human milk-based products in feeding extremely premature infants. Breastfeed. Med. 7, 29–37 (2012).
Parker, M. G. et al. Prevalence and predictors of donor milk programs among U.S. advanced neonatal care facilities. J Perinatol. 40, 672–680 (2020).
Norberg, S. et al. Cronobacter spp. in powdered infant formula. J. Food Prot. 75, 607–620 (2012).
Acknowledgements
Publication of this article was sponsored by the Necrotizing Enterocolitis (NEC) Society, Patient-Centered Outcomes Research Institute, and National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development and The Texas Children’s Hospital Neonatal Nutrition Research Program.
Author information
Authors and Affiliations
Contributions
A.B.H., J.F., C.G., J.H.K., and S.N.T. made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. All authors were involved in drafting the article or revising it critically for important intellectual content and provided final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.B.H. received grant support for unrelated studies from Prolacta Bioscience, Fresenius-Kabi, and the Gerber Foundation. J.H.K. received grant support from Mallinckrodt and Fresenius-Kabi, received consultant or advisor fees from Evolve Bioscience, Medela, Alcresta, Ferring, received lecture fees from Mead Johnson Nutrition, Abbott Nutrition, owns shares in Pediasolutions, Nicolette and Astarte Medical, served as expert witness, and holds a patent for a newborn heart rate device. S.N.T. received unrelated grant support from Allen Foundation, National Institute of Health, Mallinckrodt, and Prolacta Bioscience. J.F. and C.G. declared no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hair, A.B., Ferguson, J., Grogan, C. et al. Human milk fortification: the clinician and parent perspectives. Pediatr Res 88 (Suppl 1), 25–29 (2020). https://doi.org/10.1038/s41390-020-1076-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-1076-2