Abstract
Background
We aimed to evaluate whether serum hepcidin is a useful indicator of iron status in infants.
Methods
Term infants (n = 400) were randomized to delayed (≥180 s) or early (≤10 s) cord clamping (CC). Iron status was assessed at 4 and 12 months. In all cases with iron depletion or iron deficiency (ID) (as defined in “Methods”) (n = 30) and 97 randomly selected iron-replete infants, we analyzed hepcidin and explored its correlation to the intervention, iron status, and perinatal factors.
Results
Serum hepcidin concentrations were significantly lower in the early CC group at both time points and in ID infants at 4 months. Median (2.5th–97.5th percentile) hepcidin in non-ID infants in the delayed CC group (suggested reference) was 64.5 (10.9–142.1), 39.5 (3.5–157.7), and 32.9 (11.2–124.2) ng/mL in the cord blood and at 4 and 12 months, respectively. The value of 16 ng/mL was a threshold detecting all cases of iron depletion/ID at 4 months. No similar threshold for ID was observed at 12 months. The strongest predictor of hepcidin at both ages was ferritin.
Conclusions
Hepcidin is relevant as iron status indicator in early infancy and may be useful to detect ID. Levels <16 ng/mL at 4 months of age indicates ID.
Impact
-
Serum hepcidin is a relevant indicator of iron status in early infancy.
-
Normal reference in healthy infants is suggested in this study.
-
Serum hepcidin may be useful in clinical practice to detect iron deficiency.
Similar content being viewed by others
Introduction
Owing to rapid growth rate and high iron requirements, infants are at risk of iron deficiency (ID)1,2 As the most common single nutrient deficiency, ID is not only a lead cause of the high global prevalence of anemia but also a risk factor for impaired neurodevelopment in infants and young children.3,4,5,6 From a clinical viewpoint, the ability to evaluate iron status in individuals and populations are of great importance. However, iron metabolism undergoes a rapid, dynamic change during the first months of life, where both iron stores and erythropoiesis passes through different physiological phases, and the exact regulatory mechanisms are not fully understood.7,8,9 This dynamic physiology of iron metabolism in infants is reflected by rapid changes of traditional iron status markers, such as hemoglobin, ferritin, and transferrin saturation (TS).10,11 It is not well known which biomarkers best reflect the narrow path of normal iron status, particularly during the first 6 months of age when both ID and overload can cause potential harm. Therefore, further research regarding biomarkers and their association with iron stores and functional outcomes has been pointed out as one of the future research directions in the area.12
Hepcidin, a peptide described in the early twenty-first century, is believed to be the main iron regulatory hormone in the body.13,14,15 Binding to the iron transporter protein ferroportin, it downregulates the cellular export to the blood stream, indirectly lowering iron absorption in the intestine and iron release from cellular stores.14,16 It has been confirmed in adults that hepcidin expression decreases during ID and studies in infants have showed a close correlation to ferritin and other iron status indicators.17 However, observational studies in infants and children with ID have showed diverging results with regard to iron status assessment,17,18,19 and the use of hepcidin as a reliable iron status indicator in infancy is not fully evaluated.
In the current study, we aimed to evaluate the use of hepcidin as an iron status indicator in infants. Using stored serum samples from a previously published randomized trial of early vs. delayed cord clamping (CC) in healthy normal birth weight infants, we assessed the normal range of hepcidin in infants at 4 and 12 months of age and explored the association with traditional iron status indicators, as well as to the CC intervention.
Methods
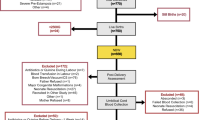
This study is a secondary analysis of a previously published randomized controlled trial including 400 newborn infants in the Hospital of Halmstad, Sweden between April 16, 2008, and May 22, 2009.20 Briefly, pregnant women were approached at the antenatal care units associated with the hospital if fulfilling the inclusion criteria (nonsmoking, healthy, and carrying a low-risk singleton pregnancy) with an expected vaginal delivery at term. The women included gave written informed consent, and when delivery was imminent, they were randomized to delayed CC at ≥180 s after delivery or to early CC before or at 10 s after birth (Fig. 1). As presented previously, the intervention allocation was partly blinded,20 but at the time of the present study, the intervention allocation was unblinded to all study staff. The trial was approved by the Regional Ethical Review Board of Lund University.
As part of the primary study protocol for the original study, iron status was assessed in cord blood and at 4 and 12 months of age including serum measures of ferritin, TS, mean cell volume (MCV), and transferrin receptor (TfR) concentration.20,21 ID at 4 and 12 months was defined as two or more iron status indicators outside the reference range (low ferritin level, low MCV, low TS, or high sTfR level) as defined in Table 1.
In the present study, a subsample of 127 infants from the original study was selected for secondary analyses of hepcidin concentrations (Fig. 1). We included all 24 cases who were diagnosed with ID at 4 months (n = 9), 12 months (n = 13), or at both these times (n = 2). In addition, we included 4 cases with iron depletion (ferritin <20 μg/L) at 4 months and 2 cases with iron depletion (ferritin <10 μg/L) at 12 months. Finally, we randomly selected another 97 infants who had complete iron status analyses performed at all three measurements but never diagnosed with ID (non-ID group). Finally, cases with CC-reactive protein (CRP) ≥5 mg/L were excluded from all analyses.
Stored serum for these 127 included cases was analyzed for C-Hepcidin-25 using enzyme-linked immunoassay (ELISA) (Human hepcidin-25, extraction-free, EIA Kit, Cat. No S-1337, Bachem, Peninsula Laboratories, San Carlos) in accordance with the manufacturer’s instructions. According to the manufacturer, this ELISA is specific for hepcidin-25. Before analysis, the serum samples were diluted 1:6 in treated human serum provided with the kit. Hepcidin-25 levels were calculated from a calibration curve with a linear measuring range near 1.5 ng/mL. Samples with coefficient of variation >15% or with levels falling outside the measuring range were re-assayed in a different dilution.
Statistical approach
The sample size of the present study was limited by funding and by the number of ID cases in this low-risk population. A pre-study power calculation showed that, with a power of 80% and a significance level of 0.05, a sample size of >100 analyzed cases would be able to detect a difference in hepcidin between the infants from the delayed and the early CC groups of 18 ng/mL at 4 months. However, hepcidin analyses showed a skewed distribution, and we chose to perform the group comparisons (early CC vs. delayed CC and ID vs. never-ID) using non-parametric Mann–Whitney test and regression analyses after log transformation. All other comparisons were performed using t test for continuous variables and chi-square test for proportions. The correlation between hepcidin and possible predictors was assessed using univariate regression. Owing to small number of subjects, we did not explore the associations in a multivariate regression models.
Results
The group characteristics of the 127 included infants are presented in Table 2. The cohort analyzed was representative of the overall study population with no significant differences compared to those not included.
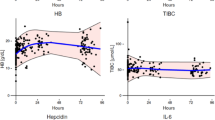
Hepcidin was successfully analyzed in 121 cord blood samples, 118 infants at 4 months, and 118 infants at 12 months. The median hepcidin (interquartile range (IQR)) was 58.0 (35.9; 76.4), 32.5 (16.4; 47.5), and 30.0 (19.0; 48.3) ng/mL at birth (cord blood), 4 months, and 12 months, respectively. The levels were significantly lower in the early CC group at both 4 and 12 months (Fig. 2). Percentiles for hepcidin concentrations in infants in the delayed CC group who never developed ID or iron depletion are presented in Table 3. This group was chosen as a reference since delayed CC has been shown to improve iron status is infants and is currently recommended by most authorities.22 The suggested normal range (2.5th–97.5th percentile) is 10.9–142.1 ng/mL at birth, 3.5–157.7 ng/mL at 4 months, and 11.2–124.2 ng/mL at 12 months.
To explore the possible predictors of hepcidin at different ages and its association with other iron status indicators, univariate linear regression models were used and are presented in Table 4. Hepcidin increased with birth weight and correlated closely to ferritin at both ages. Furthermore, at 4 months there was a close correlation to the other iron status indicators. This association was further explored in Fig. 3a, where analyzed cases with iron depletion (ferritin <20 μg/L) or ID at 4 months (n = 10) were compared to the infants from the delayed CC group who were never diagnosed with ID or iron depletion (n = 50), showing a significant difference (p < 0.001). Among ID/iron-depleted infants, 100% had a hepcidin value <16 ng/mL, while 92% of all non-ID cases had a value above that threshold. A similar threshold could not be identified at 12 months where hepcidin in the iron-deplete/ID cases ranged from 7 to 139 ng/mL compared to 6–132 ng/mL in the non-ID cases (Fig. 3b).
Discussion
Our results showed that hepcidin is sensitive to changes in iron status and a promising detector of ID in early infancy. The biomarker correlated closely to ferritin and all of the 10 iron-deficient/iron-deplete infants at 4 months had a hepcidin level below the 10th percentile of the non-anemic delayed CC group—a group that we suggest could be used as reference for normal range.
Implications on determining iron status in infants
The importance of sensitive iron status indicators cannot be underestimated. ID is considered the most common micronutrient deficiency worldwide, and it has recently been listed among the eight most common chronic diseases23 and one of the five leading causes of years lived with disability.24 ID is not only a major risk factor for anemia but also a risk factor for delayed neurocognitive development. It has been shown in several observational studies and some randomized trials that low iron availability is associated with impaired psychomotor development and increased risk of behavioral problems.6,25,26 Individual screening or prophylactic supplementation is recommended to risk groups to reduce this negative impact.8,12,27
It is common, especially in low-income countries, to screen for ID by measuring hemoglobin levels since ID is a common cause of anemia. However, recent research suggests that negative effects on brain development may occur already in early stages of ID, since the brain’s iron supply is compromised before the red cells become iron depleted.26,28,29 Thus there are strong reasons to screen infants and young children at risk for non-anemic ID. Unfortunately, there is no clear consensus of how to best assess ID in individuals or in screening programs and currently used cut-offs are diverging and are mostly not well defined.26,30 Ferritin is the most widely used iron status marker, but it is limited by a close correlation to inflammation and to the lack of correlation to functional deficiency, i.e., signs of low iron availability in iron-dependent cells. Other iron status markers include TS, TfR concentration, MCV, reticulocyte hemoglobin, and hepcidin,18,27 often used in combinations such as in the present study. Nevertheless, research regarding the specificity and sensitivity for each of these markers is limited, particularly with regard to infants. Since infants undergo a rapid physiological change in iron availability, intake, and compartments, research from adults cannot be directly extrapolated.9 Furthermore, it has been suggested that infants have an immature ability to downregulate iron absorption and that other regulatory mechanisms may exist.7,31,32
Previously published studies
The suggested normal range for serum hepcidin concentrations in adults is 0.6–23 ng/mL33 even though some research group reported higher concentrations.34 However, with regards to children during the first year of life, there are large physiological changes in iron metabolism, and there has been few studies reporting hepcidin concentrations.35,36 To our knowledge, there are only four recently published studies in healthy infants.19,36,37,38 In two of them, the number of included infants were low.19,36 Mupfadze et al. reported hepcidin in 139 Zambian infants participating in vitamin A supplementation trial.37 Only non-anemic, iron-replete infants with no inflammation were selected. Hepcidin decreased over the first year of life with median concentrations (IQR) of 9.7 (2.5–19.25), 4.5 (0.49–7.32), and 1.9 (0.73–6.17) ng/mL at 3, 6, and 12 months, respectively. In a Spanish longitudinal study, mean hepcidin in healthy infants with normal iron status (74% of those 140 included) was 44.77 (SD 1.5) at 6 months, and 54.28 ng/mL (SD 1.5) at 12 months.38 It is difficult to compare these results with each other and with our data due to the lack of a standardized laboratory method and different detection ranges. Moreover, they suggest that hepcidin levels may differ between populations, possibly the effect of differences in how iron status changes during early life, early feeding practices, and/or gene polymorphism.
Results of the present study
The present study confirms that the expression of hepcidin is sensitive to iron availability. The levels increased significantly in the infants who were subjected to delayed CC and was significantly lower in those diagnosed with ID at 4 months. The finding is in concordance with our previous results from a cohort of low birth weight infants supplemented with placebo or iron.17 Also in that trial, we found that hepcidin decreased in parallel with lower ferritin levels and that it was significantly higher at both 12 weeks and 6 months of age in those supplemented with iron.17 If there is an immaturity in infants’ ability to downregulate iron absorption, it is more likely due to suboptimal sensitivity to hepcidin in the enterocyte, e.g., on the target molecule ferroportin. Such poor sensitivity was recently suggested from a study in piglets.31
Furthermore, our results suggest that hepcidin has a high sensitivity to ID in early infancy, a characteristic that could be very beneficial in clinical practice. This conclusion was also done by Zaman et al. in a study of pregnant women. They concluded that serum hepcidin is superior to hemoglobin, serum iron, serum ferritin, TS, and total iron-binding capacity as indicator of ID anemia.39 However, our study was not powered to calculate sensitivity using receiver operating characteristic (ROC) curve analysis. It should also be underscored that, only if a gold standard such as bone marrow staining is used, could a marker of true tissue depletion be evaluated. This would, however, be ethically unacceptable. Future studies are recommended comparing ferritin, hepcidin, and other suggested markers of early ID with regard to tissue depletion or functional outcomes.
Interestingly, we did not observe the same clear sensitivity to ID at 12 months even though there was a similar correlation to ferritin and the number of ID cases were actually higher. A similar lack of sensitivity was observed by Dewan et al. who assessed children of different ages with iron deficiency anemia (IDA) and found that hepcidin was not useful to detect IDA.19 One reason could be higher prevalence of subclinical inflammation. We excluded cases with CRP ≥5 mg/L; however, there may be other cases with inflammatory signaling that interacts with the hepcidin expression. The difficulties of assessing iron status in a state of inflammation was recently reviewed and authors concluded that there is still lack of knowledge and validated indicators.19,40 Further supporting this lack of sensitivity to ID at 12 months was the inverse correlation to TS (Table 4). The finding at 4 months was more according to what should be expected.
Strengths and limitations
The strength of the present study is its well-defined cohort of healthy infants and its randomized design. The analyses of iron status using multiple biomarkers enabled important comparisons and conclusions. The low prevalence of ID in this population limited the possibilities to explore hepcidin as a marker of ID using ROC curve analysis, and as discussed above, a measure of iron using a gold standard was lacking. It should be noted that a limiting factor when discussing age-specific reference ranges for clinical use is the lack of standardized laboratory methods. We have previously shown that the EIA Kit used in the present study correlates well to a mass spectrometry but with generally higher levels.41
Conclusion
We confirmed in this study that healthy infants express hepcidin in response to iron availability and that hepcidin concentration decreases significantly during ID, at least at 4 months of age. The presented normal range of hepcidin (Table 3) could be used in future research as well as in clinical practice. We suggest that additional studies are designed to evaluate whether hepcidin could have a role in clinical practice where detection of early stages of ID in infants is of highest priority.
References
WHO. Iron Deficiency Anaemia: Assessment, Prevention and 2001 Control. A Guide for Programme Managers (World Health Organization, Geneva, 2001).
de Benoist, B., McLean, E., Egli, I. & Cogswell, M. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia (World Health Organization, Geneva, 2008).
Doom, J. R. & Georgieff, M. K. Striking while the iron is hot: understanding the biological and neurodevelopmental effects of iron deficiency to optimize intervention in early childhood. Curr. Pediatr. Rep. 2, 291–298 (2014).
Berglund, S. K. et al. Effects of iron supplementation of LBW infants on cognition and behavior at 3 years. Pediatrics 131, 47–55 (2013).
Camaschella, C. Iron deficiency. Blood 133, 30–39 (2019).
Andersson, O. et al. Effect of delayed cord clamping on neurodevelopment at 4 years of age: a randomized clinical trial. JAMA Pediatr. 169, 631–638 (2015).
Lonnerdal, B. Development of iron homeostasis in infants and young children. Am. J. Clin. Nutr. 106, 1575S–1580S (2017).
Berglund, S. & Domellof, M. Meeting iron needs for infants and children. Curr. Opin. Clin. Nutr. Metab. Care 17, 267–272 (2014).
Helman, S. L., Anderson, G. J. & Frazer, D. M. Dietary iron absorption during early postnatal life. Biometals 32, 385–393 (2019).
Saarinen, U. M. & Siimes, M. A. Developmental changes in serum iron, total iron-binding capacity, and transferrin saturation in infancy. J. Pediatr. 91, 875–877 (1977).
Saarinen, U. M. & Siimes, M. A. Serum ferritin in assessment of iron nutrition in healthy infants. Acta Paediatr. Scand. 67, 745–751 (1978).
Domellof, M. et al. Iron requirements of infants and toddlers. J. Pediatr. Gastroenterol. Nutr. 58, 119–129 (2014).
Krause, A. et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 480, 147–150 (2000).
Nemeth, E. et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 (2004).
Park, C. H., Valore, E. V., Waring, A. J. & Ganz, T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J. Biol. Chem. 276, 7806–7810 (2001).
Aschemeyer, S. et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood 131, 899–910 (2018).
Berglund, S., Lonnerdal, B., Westrup, B. & Domellof, M. Effects of iron supplementation on serum hepcidin and serum erythropoietin in low-birth-weight infants. Am. J. Clin. Nutr. 94, 1553–1561 (2011).
Ambroszkiewicz, J. et al. Serum hepcidin and soluble transferrin receptor in the assessment of iron metabolism in children on a vegetarian diet. Biol. Trace Elem. Res. 180, 182–190 (2017).
Dewan, P. et al. Serum and urinary hepcidin for diagnosing iron-deficiency anemia in under-5 children. J. Pediatr. Hematol. Oncol. 41, e216–e220 (2019).
Andersson, O., Hellstrom-Westas, L., Andersson, D. & Domellof, M. Effect of delayed versus early umbilical cord clamping on neonatal outcomes and iron status at 4 months: a randomised controlled trial. BMJ 343, d7157 (2011).
Andersson, O., Domellof, M., Andersson, D. & Hellstrom-Westas, L. Effect of delayed vs early umbilical cord clamping on iron status and neurodevelopment at age 12 months: a randomized clinical trial. JAMA Pediatr. 168, 547–554 (2014).
WHO. Guideline: Delayed Umbilical Cord Clamping for Improved Maternal and Infant Health and Nutrition Outcomes (World Health Organization, Geneva, 2014).
GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1545–1602 (2016).
GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1211–1259 (2017).
Berglund, S. K. et al. Effects of iron supplementation of low-birth-weight infants on cognition and behavior at 7 years: a randomized controlled trial. Pediatr. Res. 83, 111–118 (2018).
Georgieff, M. K., Krebs, N. F. & Cusick, S. E. The benefits and risks of iron supplementation in pregnancy and childhood. Annu. Rev. Nutr. 39, 121–146 (2019).
Baker, R. D., Greer, F. R. & Committee on Nutrition American Academy of Pediatrics. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics 126, 1040–1050 (2010).
Rao, R. et al. Metabolomic analysis of CSF indicates brain metabolic impairment precedes hematological indices of anemia in the iron-deficient infant monkey. Nutr. Neurosci. 21, 40–48 (2018).
Zamora, T. G., Guiang, S. F. 3rd, Widness, J. A. & Georgieff, M. K. Iron is prioritized to red blood cells over the brain in phlebotomized anemic newborn lambs. Pediatr. Res. 79, 922–928 (2016).
Daru, J. et al. Serum ferritin as an indicator of iron status: what do we need to know? Am. J. Clin. Nutr. 106, 1634s–1639s (2017).
Ji, P. et al. Duodenal ferroportin expression is unresponsive to dietary iron excess in nursing piglets (FS04-01-19). Curr. Dev. Nutr. 3, nzz048.FS04-01-19 (2019).
Domellöf, M. L. B., Abrams, S. A. & Hernell, O. Iron absorption in breast-fed infants: effects of age, iron status, iron supplements, and complementary foods. Am. J. Clin. Nutr. 76, 198–204 (2002).
Galesloot, T. E. V. S. et al. Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood 117, e218–e225 (2011). 2011.
Ganz, T. O. G., Girelli, D., Nemeth, E. & Westerman, M. Immunoassay for human serum hepcidin. Blood 112, 4292–4297 (2008).
Kumar, S. B. P., Jain, R. & Bharti, B. Plasma hepcidin levels in healthy children: review of current literature high. J. Pediatr. Hematol. Oncol. 41, 238–242 (2019).
Donker, A. E. et al. Standardized serum hepcidin values in Dutch children: set point relative to body iron changes during childhood. Pediatr. Blood Cancer 67, e28038 (2020).
Mupfudze, T. G. et al. Hepcidin decreases over the first year of life in healthy African infants. Br. J. Haematol. 164, 150–153 (2014).
Aranda, N. B. C. et al. Serum hepcidin levels, iron status, and HFE gene alterations during the first year of life in healthy Spanish infants. Ann. Hematol. 97, 1071–1080 (2018).
Zaman, B., Rasool, S., Jasim, S. & Abdulah, D. Hepcidin as a diagnostic biomarker of iron deficiency anemia during pregnancy. J. Matern. Fetal Neonatal. Med. https://doi.org/10.1080/14767058.2019.1635112 (2019).
Suchdev, P. S. et al. Assessment of iron status in settings of inflammation: challenges and potential approaches. Am. J. Clin. Nutr. 106, 1626S–1633S (2017).
Uijterschout, L. et al. Serum hepcidin measured by immunochemical and mass-spectrometric methods and their correlation with iron status indicators in healthy children aged 0.5-3 y. Pediatr. Res. 76, 409–414 (2014).
Acknowledgements
The secondary study was supported by Swedish Research Council, Vetenskapsrådet (2019–01005), Knut and Alice Wallenberg foundation, and Västerbotten County Council.
Author information
Authors and Affiliations
Contributions
M.D. and O.A. designed the original study; O.A. collected the data. All authors contributed to the secondary study design, analysis, and/or interpretation of data. S.K.B. and A.M.C. drafted the article. All authors revised it critically and approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent
The research was approved by the Regional Ethical Review Board of Lund University and included pregnant women gave written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Berglund, S.K., Chmielewska, A.M., Domellöf, M. et al. Hepcidin is a relevant iron status indicator in infancy: results from a randomized trial of early vs. delayed cord clamping. Pediatr Res 89, 1216–1221 (2021). https://doi.org/10.1038/s41390-020-1045-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-1045-9