Abstract
Background
Small-for-gestational-age (SGA) neonates are at a higher risk of adult-onset metabolic disorders because of fetal programming in the presence of growth restriction. Nephrogenesis may also be affected in fetal growth restriction. This study hypothesized that urinary podocalyxin levels, a marker of nephrogenesis, would be lower among preterm SGA neonates as compared to appropriate-for-gestational-age (AGA) controls.
Methods
This cross-sectional study enrolled gestation-matched SGA (n = 90) and AGA (n = 45) neonates born at 260–366 weeks of gestation. The SGA group comprised of 45 neonates with birth weight between 3rd and 10th centile and 45 neonates with birth weight <3rd centile. The primary outcome of the study was the difference in urinary podocalyxin levels between SGA and AGA neonates. Glomerular and tubular functions were also assessed.
Results
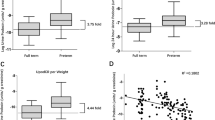
Urinary podocalyxin levels were similar in SGA and AGA neonates (ng/mg of creatinine; median [interquartile range]: 28.7 [4.8–70.2] vs. 18.7 [3.1–55.9]), P value 0.14). No correlation was observed between birth weight centile and urinary podocalyxin levels (r: −0.06). Glomerular filtration rate, fractional excretion of sodium, and serum β-2-microglobulin levels were comparable across the study groups.
Conclusions
Glomerular development as assessed by urinary podocalyxin levels and renal functions are comparable in SGA and AGA preterm neonates.
Impact
-
Neonates born with fetal growth restriction are at a higher risk of adult-onset metabolic disorders because of fetal programming.
-
This cross-sectional study investigated the effect of presence and severity of fetal growth restriction on glomerular development by measuring urinary podocalyxin levels in preterm infants.
-
This study did not observe any effect of the presence or severity of fetal growth restriction on urinary podocalyxin levels and other markers of glomerular and renal tubular functions.
Similar content being viewed by others
Introduction
Worldwide, low birth weight (LBW) has been recognized as a significant public health problem because of its association with a plethora of short- and long-term consequences.1 More than 20 million LBW babies are born each year worldwide, with India contributing to 42% of the global burden, single largest for any country. In India, around two-thirds of LBW births are attributable to fetal growth restriction, resulting in the birth of small-for-gestational-age (SGA) neonates.2 Apart from the higher risk of neonatal death and morbidities, SGA neonates are also predisposed to a variety of adult-onset diseases in later life due to aberrant fetal programing.3,4,5
Non-communicable diseases have replaced communicable diseases as a major cause of mortality worldwide.6 Recent findings from the Global Burden of Diseases study have highlighted chronic kidney disease (CKD) as one of the major non-communicable diseases.7 CKD is a key determinant of poor health outcomes for other major non-communicable diseases and has a risk multiplier effect on cardiovascular diseases. Apart from genetic predisposition and environmental exposures, fetal development is also being recognized as an important modulator of the risk of developing non-communicable diseases including CKD.8,9 In a normally developing fetus, nephrogenesis continues till 36 weeks of gestation.9 In the case of preterm birth, nephrogenesis may continue after birth if there is no acute kidney injury. There is no addition to the number of nephrons after birth at full-term gestation. More than half of all nephrons develop during the third trimester of pregnancy, the period when fetal growth restriction is most likely to set in. It has been hypothesized that if the fetus is affected by growth restriction, the resultant developmental programming may lead to a decrease in the final number of nephrons.10,11 Decreased glomerular surface area, in turn, may result in the decreased capacity of kidneys to excrete sodium and gradual development of glomerular sclerosis. With increasing age, this can lead to the development of hypertension and CKD. Ecological data shows that the incidence of hypertension and CKD is high in poorer population segments that also have a higher incidence of LBW.12
Most of the research on the effect of fetal growth restriction on renal structure and function has been conducted in animal models.10,13,14,15 These animal studies support the hypothesis that fetal deprivation affects the nephron number. Demonstration of the number of nephrons in humans is not possible unless an autopsy is performed. Determination of renal mass and renal size is not a good marker of nephron number as the remaining nephrons hypertrophy resulting in the normalization of renal size. Therefore, ultrastructural studies are needed to determine the number of nephrons.15 As no in vivo method exists to determine the nephron number, there is a need for a biomarker for early detection of alteration in nephrogenesis. This can help in predicting which SGA neonates are at risk of developing hypertension and CKD in later life. Podocalyxin (PCX), a sialoprotein present on podocytes, has been demonstrated to be a marker of nephrogenesis. Urinary levels of PCX measured at birth in premature neonates are higher than in term neonates reflecting ongoing nephrogenesis.16,17 This could help us to recognize the babies at risk of developing CKD in later life. The present study was planned with the hypothesis that the urinary PCX levels, a marker of nephrogenesis, would be lower among preterm SGA neonates as compared to gestation-matched appropriate-for-gestation-age (AGA) controls. We also planned to evaluate the association between the severity of fetal growth restriction as assessed by birth weight centile and urinary PCX levels, and to compare glomerular and tubular functions at birth in SGA and AGA neonates.
Materials and methods
This cross-sectional study was conducted from 1 May 2018 to 31 July 2019 in the Department of Neonatology in collaboration with the Department of Biochemistry at Government Medical College and Hospital, Chandigarh, India. The study protocol was approved by the Institute Ethics Committee and written informed consent was obtained from either parent of the neonate before enrollment.
Study subjects
Preterm neonates born in the hospital between 26+0 and 36+6 weeks of gestation were eligible for enrollment. Neonates were enrolled in the following two groups:
-
1.
SGA group included neonates with birth weight <10th percentile for gender and gestation as per Intergrowth-21 size at birth growth standards.18 This group comprised of an equal number of neonates with birth weight between 3rd and 10th centile and birth weight below 3rd centile.
-
2.
Control group included neonates with birth weight between 25th and 90th centile for gender and gestation.18 Neonates between the 10th and 25th centile were not included in the control group as some of these neonates have borderline fetal growth restriction and have morphological features of growth restriction, but do not fulfill the statistical definition (birth weight <10th centile weight). To avoid contamination, neonates with birth weight >25th centile were enrolled in the control group. AGA neonates were enrolled as control subjects.
Neonates with a major congenital malformation, fetal renal pelvic dilatation, or any renal anomaly detected in the antenatal period, significant perinatal asphyxia defined as the need of bag and mask ventilation for at least 30 s, history of nephrotoxic drug intake by mother during pregnancy, anuria from birth till 48 h of age, shock defined as the need of inotropic support in first 48 h, and monochorionic–monoamniotic twins were excluded.
Gestation at birth was calculated from the date of the last menstrual period. If the latter was not available or the mother was not sure about the dates, gestation was assigned by first-trimester ultrasound. If the first-trimester ultrasound was not available, gestation was assigned by Expanded New Ballard Scoring.19
Potentially eligible neonates were identified from the labor room birth register. Of potentially eligible neonates, the least common were those born with birth weight <3rd centile. Therefore, the enrollment process hinged on the identification of these neonates. Once an SGA neonate with birth weight <3rd centile baby was enrolled, next born gestation-matched (±1 week) neonate with birth weight between 3rd and 10th centile, and the next born gestation-matched (±1 week) AGA neonate were enrolled. The following information was collected about enrolled neonates: mode of delivery, birth weight, gender, period of gestation, intrauterine growth status, need of resuscitation, and Apgar scores at 1 and 5 min of age. Antenatal information about pregnancy was collected by screening the mother’s health records and by an interview with the mother. Birth weight was measured with a weighing scale with 10 g sensitivity. The accuracy of the weighing scale was checked against standard weight every week. The length of the neonate was measured using an infantometer. Head and chest circumference were measured using a non-stretchable plastic measuring tape with 0.1 cm accuracy. Z-score and Ponderal index were calculated from the anthropometric measurements.
Study measurements
Two milliliters of blood was collected from a peripheral vein of the neonate between 24 and 48 h of age. Serum was separated and divided into two aliquots. One aliquot was processed to measure levels of β-2-microglobulin and the other for urea, creatinine, and sodium. Fractional excretion of sodium and creatinine clearance was calculated. A urine collecting bag was applied at perineum of the neonate for non-invasive collection of urine. Five milliliters of urine passed within 48 h of birth was collected. After centrifugation, the sample was divided into three aliquots. One was analyzed for urine creatinine and urine sodium levels. Two aliquots were stored at −80 °C for measurement of PCX levels.
PCX measurement
One day before PCX measurement, urine samples were shifted to a freezer with −20 °C temperature. On the day of measurement, urinary samples were thawed at room temperature for 2 h and then analyzed for PCX levels. PCX levels were done using the enzyme-linked immunosorbent assay (ELISA) technique (sandwich ELISA, RayBio® Human Podocalyxin ELISA kit: GA, USA). PCX measurement was done as per the manufacturer’s instructions and the kit had a lower detection limit of assay of 22 pg/mL. The resultant levels were converted into ng per mg of creatinine.
Study outcomes
The primary outcome of the study was the mean difference (with a 95% confidence interval) in urinary PCX levels between SGA and control groups.
Secondary outcomes included:
-
SGA vs. control group: Mean difference and 95% confidence interval in glomerular filtration rate, fractional excretion of sodium, serum β-2-microglobulin and serum creatinine.
-
Three-group comparison between AGA neonates, SGA neonates with birth weight between 3rd and 10th centile and SGA neonates with a birth weight below 3rd centile: mean difference and 95% confidence interval in urinary PCX, creatinine clearance, fractional excretion of sodium, serum β-2-microglobulin and serum creatinine.
Statistical analysis
In a previous study, urinary PCX levels in a mixed cohort of preterm neonates were observed to be 188.3 ± 134.8 ng/mg of creatinine.17 A sample size of 90 in the SGA and 45 in the control group was calculated to be able to detect a decrease in PCX concentration by 50% with a power of 80% and a two-sided α error of 0.05. To ensure representation across different gestation ages, recruitment was done in three gestation strata: 26+0 to 31+6 weeks, 32+0 to 33+6 weeks, and 34+0 to 36+6 weeks.
Study data were collected using a pre-tested proforma. Data were entered in an electronic data entry form in the RedCap program hosted on a local server. Inbuilt validation checks were used to ensure the accuracy and completeness of data entry. Data were imported into and analyzed using Stata 13.1 statistical software. Continuous variables were expressed as mean and standard deviation if normally distributed and as median and interquartile range if not normally distributed. Categorical variables were expressed as number and proportion. Continuous variables were compared using t test (two-group comparison) or analysis of variance test (three-group comparison) if normally distributed data and by Mann–Whitney U test (two-group comparison) or Kruskal–Wallis (three-group comparison) if not normally distributed. Friedman’s test was used for the matched analysis of the secondary outcomes across the three groups. Bonferroni correction was applied for multiple comparisons. Categorical variables were analyzed using χ2 test or Fisher’s exact test. Correlation and its 95% confidence interval were judged by calculating Spearman’s or Pearson’s correlation coefficient. A generalized linear model with the log link function was used to evaluate the variables affecting the urinary PCX levels. There were no missing data.
Results
Study flow
A total of 7764 newborns were born alive during the study period, of which 2709 neonates were born at a gestational age between 260 and 366 weeks (Fig. 1). As per the estimated sample size, 135 neonates were included in the study—90 in the SGA group and 45 in the control group.
Baseline variables
Important baseline maternal variables were comparable in two study groups (Table 1). Overall mean gestational age at birth was 34.2 ± 1.6 weeks with adequate matching (as per study design) across the study groups. Birth weight, length, head circumference, and Ponderal index were lower in the SGA group. Neonates in either study group were equally likely to have important antenatal maternal complications, have received antenatal steroids, or be born by cesarean delivery. Less than 10% neonates needed any resuscitation at birth and Apgar scores at 1 and 5 min were comparable. Nine (10%) neonates in the SGA and four (8.8%) neonates in the control group needed admission to neonatal intensive care unit (NICU). Indication of admission to the NICU was comparable for the study groups (data not shown). All enrolled neonates were discharged alive from the hospital.
Study outcomes
Primary outcome
The mean age of neonates at the time of collection of samples for outcome measurement was comparable in the two study groups. There was no significant difference in the urinary PCX levels in the SGA and control neonates (ng/mg of creatinine; median [interquartile range]: 28.7 [4.8–70.2] vs. 18.7 [3.1–55.9]), P value 0.14 (Table 2, Fig. 2). Further, there was no association between the severity of growth restriction and urinary PCX levels (Table 2, P values not significant) and no correlation between birth weight centile and urinary PCX values (Spearman’s correlation coefficient: −0.06, P value: 0.50). Urinary PCX levels were comparable in the two groups even after adjusting for the effect of the difference in birth weight in the two groups.
Secondary outcomes
Glomerular and tubular functions as assessed by fractional excretion of sodium, glomerular filtration rate (calculated as creatinine clearance rate), serum β-2-microglobulin, and serum creatinine were comparable across the study groups (Table 2).
Discussion
The low birth weight being a significant public health problem in India and the fact that two-thirds of the LBW neonates are the result of fetal growth restriction, this study was planned to evaluate renal development and function in SGA neonates. The study was based on the hypothesis that if a fetus is affected by growth restriction, the resultant developmental programming may lead to a decrease in the final number of nephrons. The study results indicate that glomerular development as measured by urinary levels of glomerular protein PCX is not affected by the presence or severity of growth restriction. The tubular function was also found to be preserved.
Only preterm neonates were enrolled in this study. As nephrogenesis is completed by 36 weeks of gestation, urinary PCX levels decrease at term gestation, and therefore, may not be useful to study the effect of fetal growth restriction. Further, to prevent bias due to gestation-related change in renal development, we enrolled gestation-matched neonates in the control group. We also excluded conditions like significant perinatal asphyxia and shock in the first 48 h of life, which may lead to glomerular injury and increase the urinary PCX levels.20
During glomerular development, PCX appears just before the podocyte foot process and slit diaphragm formation. PCX is released into extracellular space as a soluble cleaved fragment of ectodomain by membrane metalloproteinases. Since the apical surface of the podocytes faces the urinary space, urinary PCX at birth might be a marker of nephron formation.21,22 However, no threshold has been defined to characterize normal nephron numbers or PCX levels. In a study published in 2017, Hayashi et al.17 measured PCX, β-2-microglobulin, and N-acetyl-β-d-glucosaminidase in the urine of preterm neonates at birth and at 37–39 weeks of postmenstrual age to evaluate the usefulness of PCX as a biomarker of nephrogenesis, glomerular function, and podocyte injury.17 PCX was detectable in all urine samples, with urinary levels at birth (188.3 ± 134.8 ng/mg creatinine) being higher than the previously reported reference value of 62.8 ± 4.4 ng/mg creatinine in adults. The authors proposed that higher levels of PCX at the time of birth were due to ongoing nephrogenesis and/or renal injury during complications of delivery.17,20 The authors also hypothesized that lower levels of PCX at 37–39 weeks of corrected age reflected an adaptation of renal function to the extrauterine environment. In this study, 22.8% of enrolled neonates had fetal growth restriction. However, authors did not provide separate data about this group of neonates. Like the study by Hayashi et al.,17 we have also observed wide variation in the urinary PCX levels. This may be due to a wide range of gestation of enrolled neonates and a wide range of the normal number of total nephrons in neonates. More recently, in 2019, Aly et al.23 have reported deranged glomerular but comparable tubular functions in SGA preterm neonates in comparison to AGA preterm neonates. In this study, 50 (25 SGA and 25 AGA) infants were included. On the day of life 1, the SGA group had higher serum sodium, serum urea, and urinary creatinine. On day 5, SGA infants had a significant increase in serum creatinine (P = 0.04). Urinary N-acetyl-β-d-glucosaminidase and fractional excretion of sodium were comparable among the two groups on days 1 and 5. However, in our study, both tubular (fractional excretion of sodium, β-2-microglobulin) and glomerular (serum creatinine, creatinine clearance, PCX) functions were comparable in SGA and AGA neonates.
Our study has many strengths. To the best of our knowledge, this is the first study investigating the effect of presence and severity of fetal growth restriction on glomerular and tubular development and function. We prevented possible confounding by matching for gestation and excluding neonates with significant perinatal insult. We report a wide repertoire of renal functions by measuring creatinine clearance, fractional excretion of sodium, serum creatinine, and serum β-2-microglobulin. However, we did not measure the size of kidneys by imaging or nephron number by renal biopsy. Latter would have needed an invasive procedure that was not justified without any preliminary evidence of the effect of fetal growth restriction on renal development. Our study was powered to detect at least a 50% reduction in urinary PCX levels, and we cannot exclude a smaller effect size. Last, we used size at birth as a proxy of fetal growth restriction to enroll neonates in the study. A better subject selection strategy would be the enrollment of pregnant women in which fetal growth restriction has been identified based on serial changes in the fetal biometry. However, we believe that neonates with birth weight <3rd centile are unlikely to be just constitutionally small and therefore have presented a secondary analysis based on birth weight percentile value. No observation of a “dose–response” relationship between the birth weight percentile and urinary PCX levels further strengthens our conclusion that fetal growth restriction does not affect the nephrogenesis.
To conclude, this study did not observe any effect of the presence or severity of fetal growth restriction on glomerular development or renal functions. The results of this study are important given the high prevalence of LBW in India. It would be important to replicate the results of the study in larger cohorts. Long-term measurement of renal functions should be part of such a study. This study also highlights the need for a better biomarker of intrauterine nephrogenesis and its correlation with fetal growth restriction to reflect the fetal origin of adult diseases.
References
Lee, A. C. et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob. Health 1, e26–e36 (2013).
Public Health Foundation of India. State of India’s Newborns https://www.newbornwhocc.org/SOIN_PRINTED%2014-9-2014.pdf (2014).
Barker, D. J. P. The origins of the developmental origins theory. J. Intern. Med. 261, 412–417 (2007).
Malhotra, A. et al. Neonatal morbidities of fetal growth restriction: pathophysiology and impact. Front. Endocrinol. 10, 55 (2019).
Sharma, D., Farahbakhsh, N., Shastri, S. & Sharma, P. Intrauterine growth restriction—part 2. J. Matern. Fetal Neonatal Med. 29, 4037–4048 (2016).
WHO. Global action plan for the prevention and control of NCDs 2013–2020. WHO http://www.who.int/nmh/events/ncd_action_plan/en/ (2019).
Wang, H. et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1459–1544 (2016).
Low Birth Weight and Nephron Number Working Group. The impact of kidney development on the life course: a consensus document for action. Nephron 136, 3–49 (2017).
Rosenblum, S., Pal, A. & Reidy, K. Renal development in the fetus and premature infant. Semin. Fetal Neonatal Med. 22, 58–66 (2017).
Hinchliffe, S. A., Lynch, M. R., Sargent, P. H., Howard, C. V. & Van Velzen, D. The effect of intrauterine growth retardation on the development of renal nephrons. Br. J. Obstet. Gynaecol. 99, 296–301 (1992).
Luyckx, V. A. & Brenner, B. M. Birth weight, malnutrition and kidney-associated outcomes-a global concern. Nat. Rev. Nephrol. 11, 135–149 (2015).
Lackland, D. T., Bendall, H. E., Osmond, C., Egan, B. M. & Barker, D. J. Low birth weights contribute to high rates of early-onset chronic renal failure in the Southeastern United States. Arch. Intern. Med. 160, 1472–1476 (2000).
Bauer, R. et al. Altered renal function in growth-restricted newborn piglets. Pediatr. Nephrol. Berl. Ger. 14, 735–739 (2000).
He, X. et al. Apoptosis in the kidneys of rats that experienced intrauterine growth restriction. Nephrology (Carlton, Vic.) 20, 34–39 (2015).
Hughson, M., Farris, A. B., Douglas-Denton, R., Hoy, W. E. & Bertram, J. F. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 63, 2113–2122 (2003).
Kerjaschki, D., Sharkey, D. J. & Farquhar, M. G. Identification and characterization of podocalyxin-the major sialoprotein of the renal glomerular epithelial cell. J. Cell Biol. 98, 1591–1596 (1984).
Hayashi, T. et al. Urinary podocalyxin as a possible novel marker of intrauterine nephrogenesis and extrauterine podocyte injury. Pediatr. Nephrol. Berl. Ger. https://doi.org/10.1007/s00467-017-3676-6 (2017).
Villar, J. et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet (London, England) 384, 857–868 (2014).
Ballard, J. L. et al. New Ballard Score, expanded to include extremely premature infants. J. Pediatr. 119, 417–423 (1991).
Tóth-Heyn, P., Drukker, A. & Guignard, J. P. The stressed neonatal kidney: from pathophysiology to clinical management of neonatal vasomotor nephropathy. Pediatr. Nephrol. Berl. Ger. 14, 227–239 (2000).
Fernández, D. et al. Release of podocalyxin into the extracellular space. Role metalloproteinases. Biochim. Biophys. Acta 1813, 1504–1510 (2011).
Nielsen, J. S. & McNagny, K. M. The role of podocalyxin in health and disease. J. Am. Soc. Nephrol. 20, 1669–1676 (2009).
Aly, H. et al. Renal function in small for gestational age preterm infants. J. Perinatol. https://doi.org/10.1038/s41372-019-0431-9 (2019).
Acknowledgements
We thank the Indian Council of Medical Research, New Delhi for providing Postgraduate thesis grant.
Author information
Authors and Affiliations
Contributions
A.S., D.C., J.K., V.M., and S.J. conceptualized and designed the study. A.S. and D.C. collected and analyzed the data. A.S., D.C., and J.K. drafted the initial manuscript. D.C. supervised the study. A.S., D.C., J.K., V.M., and S.J. reviewed and revised the manuscript critically. All authors approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Written informed consent was obtained from either parent of the neonate before enrollment.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Siddiqui, A., Chawla, D., Kaur, J. et al. Effect of fetal growth restriction on urinary podocalyxin levels at birth in preterm neonates. Pediatr Res 89, 962–967 (2021). https://doi.org/10.1038/s41390-020-0987-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0987-2