Abstract
Background
Providing optimal pain relief is a challenging task when caring for premature infants. The aim of this study was to compare the long-term cognitive, motor, and behavioral outcomes of preterm infants before and after the implementation of a pain and sedation protocol. In addition, we investigated whether the increased opiate administration resulting after the implementation process had an impact on these outcomes.
Methods
Cognitive outcomes were evaluated using the Kaufman Assessment Battery for Children (KABC), neuromotor examinations were based on Amiel-Tison, and behavioral outcomes were assessed using the parent-reported Child Behavior Checklist (CBCL).
Results
One hundred extremely preterm infants were included in the study (control group, n = 53; intervention group, n = 47). No significant differences were found in cognitive and motor outcomes at preschool age. However, every increase in the cumulative opiate exposure for each 100 mg/kg was weakly significantly associated with a higher risk for autism spectrum features (adjusted odds ratio (aOR) = 1.822, 95% confidence interval (CI) [1.231–2.697]; P = 0.03) and withdrawn behavior (aOR = 1.822, 95% CI [1.231–2.697]; P = 0.03) at preschool age.
Conclusion
Increased neonatal cumulative opiate exposure did not alter cognitive and motor outcomes but may represent a risk factor for autism spectrum and withdrawn behavior at preschool age.
Impact
-
The implementation of a protocol for the management of pain and sedation in preterm infants resulted in increased cumulative opiate exposure.
-
Our study adds further evidence that increased neonatal opiate exposure did not alter cognitive and motor outcomes but may yield a potential risk factor for autism spectrum disorders and withdrawn behavior at preschool age.
-
A vigilant use of opiates is recommended.
-
Further studies are needed looking for novel pain management strategies and drugs providing optimal pain relief with minimal neurotoxicity.
Similar content being viewed by others
Introduction
Preterm neonates admitted to the neonatal intensive care unit (NICU) are repeatedly exposed to painful procedures, most of them still performed without the use of adequate analgesia.1 Exposure to repeated stressful and painful procedures has been associated with several adverse outcomes, such as altered brain development,2,3 internalizing symptoms,4 and alterations in pain processing and pain sensitivity.5
Consequently, pain management in the NICU often includes opiate therapy. Concerns have been raised regarding the safety of opiates in preterm infants. Multiple lines of evidence suggest that early opiate exposure in rodents result in altered dendritic architecture, increased neuronal apoptosis,6 and behavioral and motor difficulties.7 However, translation of results of animal studies to humans is difficult and results of clinical trials are controversial.8,9
Since both conditions of over-sedation and excessive pain are related to a risk for worse neurodevelopmental outcomes, the NICU staff is challenged with regard to the appropriate pain-relieving treatment. To render the interpretation of patient’s pain more objective among different raters working in the NICU, international guidelines recommend the use of pain and sedation scales.10
Our research group recently reported that the implementation of a protocol to manage and treat neonatal pain and sedation using opiates at two NICUs at the Medical University of Vienna resulted not only in an improvement of pain and sedation management, but also in an increased opiate administration.11 We therefore examined the neurodevelopmental outcome of study patients at the age of 1 year12 and 3 years.13 Since the age of 5 years represent an important stage of cognitive, motor, and behavioral development, data were collected within the last follow-up appointment provided by our follow-up clinic at preschool age (at the age of 5–6 years). The aim of the study was to examine whether the implementation of a protocol for the management of pain and sedation had an impact on cognitive, motor, and behavioral outcomes in extremely preterm infants at preschool age. Furthermore, we evaluated whether increased cumulative opiate exposure resulting from the implementation of the protocol12 was associated with worse cognitive, motor, and behavioral outcomes at preschool age.
Methods
Setting
This single-center observational cohort study was performed at the Division of Neonatology, Pediatric Intensive Care and Neuropediatrics at the Medical University of Vienna, a tertiary perinatal center admitting an average of 900 neonates per year, including about 180 very low birth weight infants. The institutional ethics review board approved the study (EN: 1064/2018).
Patients
In 2010, the Neonatal Pain, Agitation and Sedation Scale (N-PASS) and the associated Vienna Protocol for Neonatal Pain and Sedation (V-PNPS) were implemented at two NICUs of the Medical University of Vienna.11 We evaluated all children assessed and treated using the N-PASS and the V-PNPS 1 year after the implementation process with a historical group of children belonging to the era before the implementation process at preschool age. All patients born at the Medical University of Vienna between 2009 and 2011 prior to 28 week of gestational age and weighing <1500 g, without any chromosomal aberration or congenital malformation, were eligible for inclusion in this study.
Cumulative opiate exposure
Infants were treated according to our V-PNPS using continuous, as well as bolus intravenous morphine or fentanyl. To summarize the use of both drugs in one cumulative dose, a morphine equivalent dose was calculated using the following formula: morphine equivalent dosage = fentanyl dose × 100.14
Follow-up assessment
Neurodevelopmental assessment at preschool age was performed in the follow-up clinic of the Department of Pediatrics and Adolescent Medicine and included neuromotor examinations based on the Amiel-Tison neurological assessment tool,15 cognitive assessment using the Kaufman Assessment Battery for Children (KABC),16 and behavior assessment using the parent-reported Child Behavior Checklist (CBCL).17
In addition, medical data were collected including hearing and visual impairment as well as growth parameters, including weight, height, and head circumference. Medical examination was performed on the same day as cognitive, motor, and behavioral assessment.
Cognitive assessment (KABC)
The KABC is a clinical instrument for assessing cognitive development. A German version was used in the study using age-specific reference data from German-speaking children including Germany, Switzerland, and Austria.16 A more recent version of the KABC, the KABC-II has been published in the German version by Melchers and Melchers.18 Both KABC scales were designed and validated by the same authors and share the same scientific background. However, some subscales were added in the new version. Only subscales included in both versions were used for statistical analysis: simultaneous processing scale and sequential processing scale.
Motor assessment (Amiel-Tison)
Motor function was assessed according to the standardized neurologic examination proposed by Amiel-Tison,15 including information regarding gross motor skills, fine motor skills, muscle tone, and reflexes. Information about gross motor skills, fine motor skills, muscle tone, and reflexes were extracted from the medical charts and were classified as normal vs abnormal.
Behavioral assessment (CBCL)
Behavior was assessed using the parent-reported CBCL.17 The CBCL is a questionnaire for parents revealing behavioral problems of children and adolescents. In the CBCL/1.5–5, two evaluation methods are available. In the first evaluation method, questions are grouped in seven empirically based problem scales including emotionally reactive, anxious/depressed, aggressive behavior, attention problems, somatic complaints, sleep problems, and withdrawn.
The second evaluation approach is based on the DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition).19 Five DSM-5-oriented scales are provided in the CBCL/1.5–5 including depressive problems, anxiety problems, autism spectrum features, attention deficit/hyperactivity problems, and oppositional defiant problems.
The questionnaire takes 15 min to complete. T values can be calculated for the evaluation. Furthermore, cut-off values for the individual T values can be defined. While for all problem scales and DSM-5-oriented scales, cut-off values >67 points were defined as clinically relevant, for internalizing, externalizing, and total problems cut-off values >63 points were defined as clinically relevant.17
Statistical analysis
Baseline characteristics, outcomes, and population characteristics at follow-up before and after the intervention, were compared using a t test for independent samples for normally distributed continuous outcomes. For non-normally distributed continuous outcomes, we used the Mann–Whitney test. Categorical variables between the groups were tested for significance using the chi-square test.
To control for confounding factors, multivariable linear regression was performed for the analysis of risk factors for the prediction of KABC scores at preschool age. Adjusted odds ratios (aORs) and 95% confidence interval (CI) for the prediction of gross and fine motor outcomes as well as for the prediction of clinically apparent CBCL scores were calculated using firth logistic regression, due to complete separation of the data that led to an infinite maximum likelihood estimate for these variables.20 Both multivariable and firth logistic regression analysis included an adjustment for the following risk factors: group assignment, sex, postmenstrual age, cumulative opiate exposure, intraventricular hemorrhage (IVH) grade ≥3/4, retinopathy of prematurity (ROP) grade ≥3/4, necrotizing enterocolitis (NEC) requiring surgery, and bronchopulmonary dysplasia (BPD) defined as oxygen at 36 weeks corrected age. Statistical analysis was performed using SPSS 24 for Mac (IBM Corporation, Armonk, NY, USA). Firth logistic regression was done using the R statistical software (R Core Team). A power of 70% was obtained in our post hoc power analysis of our logistic regression model performed with G*Power.21 Conclusion and interpretation of data was derived from our regression model and not from raw data (which are not adjusted for other medical conditions). A P value of <0.05 was considered as statistically significant.
Results
Patients
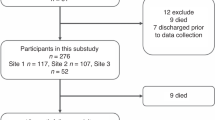
For the follow-up examination at preschool age, 100 patients were included in the analysis. An overview of infants lost to follow-up and returned to follow-up is provided in Fig. 1. Compared to the previous follow-up examination at the age of 3 years,13 20 neonates were lost to follow-up (control group, n = 11; intervention group, n = 9) and 6 additional neonates returned to follow-up (control group, n = 3; intervention group, n = 3) at 5–6 years of age (Fig. 1, Supplementary Fig. S1, Supplementary Table S1). This is in line with the literature reporting similar dropout ratios.22 Table 1 shows the baseline population characteristics, intensive care therapy, and in-hospital outcomes. There were no significant differences between the control and the intervention groups regarding population characteristics and in-hospital outcomes. However, cumulative opiate exposure was significantly higher in the intervention group when compared with the control group (48.8 (1.3–423.7) mg/kg vs 5.5 (0.8–29.8) mg/kg; P = 0.04; Table 1). No significant differences were observed in age, growth parameters, and visual and hearing impairment at preschool age (Table 1).
While green pictograms indicate infants present at the examination, red pictograms illustrate that infants were lost to follow-up at the examination. In addition, infants in both yellow and red illustrate infants who were lost to follow-up at the 1-year examination and returned to follow-up at the 5-year examination.
Cognitive, motor, and behavioral outcomes at preschool age
When comparing means of KABC scores between the intervention and control group at preschool age, no significant differences were found in the sequential processing scale (91.6 ± 19.4 vs 89.6 ± 17.0; P = 0.59) and simultaneous processing scale (97.1 ± 17.8 vs 91.6 ± 18.1; P = 0.13; Supplementary Table S2). Moreover, no significant differences were observed regarding the occurrence of motor abnormalities, including gross motor function, fine motor function, muscle tone, and reflexes at preschool age (Supplementary Table S2). Despite some missing data in the behavioral outcomes (intervention group, n = 5; control group, n = 7), oppositional defiant problems (0 vs 4; P = 0.05) and sleep problems (0 vs 4; P = 0.05) were significantly more frequent in the control group than in the intervention group (Supplementary Table S2). Yet, this was true only when looking at raw data not adjusted for other medical conditions.
Subgroup analysis was performed considering infants who did receive opiates vs infants who did not receive opiates in the neonatal period (Supplementary Tables S3 and S4 and Supplementary Fig. S2). When comparing infants regarding their opiate exposure, infants in the intervention group who did not receive opiates had significantly higher simultaneous processing scores (101.4 ± 14.2 vs 89.4 ± 21.0; P = 0.02) and higher frequency of fine motor abnormalities (6 vs 11; P = 0.01) when compared with infants who did receive opiates (Supplementary Table S3). However, this was not adjusted for other medical conditions.
Results of multivariable linear regression analysis of risk factors to predict KABC scores at preschool are provided in Table 2. Severe IVH and BPD were both predictive for lower sequential (P = 0.001 and P = 0.007, respectively) and simultaneous processing scale (P = 0.02 and P = 0.02, respectively) scores. In addition, high-grade ROP was predictive for lower sequential processing scale (P = 0.001) scores. Moreover, male sex was a risk factor for lower simultaneous processing scale (P = 0.009) scores. Higher postmenstrual age was predictive for higher sequential processing (P = 0.05) and simultaneous processing scale (P = 0.002) scores. Group assignment, NEC requiring surgery, and cumulative opiate exposure had no influence on prediction of KABC scores at preschool age (Table 2).
Firth logistic regression analysis of risk factors for the prediction of gross motor and fine motor skills at preschool age is provided in Table 3. Infants who developed high-grade IVH postnatally had a 14 times higher risk to develop altered gross motor (aOR = 14.498; 95% CI [2.054–102.322]; P = 0.002) and fine motor function (aOR = 14.910; 95% CI [0.591–376.200]; P = 0.03) at preschool age. Furthermore, occurrence of high-grade ROP after birth was associated with a sixfold higher risk of developing impaired fine motor function (aOR = 6.068; 95% CI [1.390–26.494]; P = 0.01). While male sex (aOR = 6.740; 95% CI [1.926–23.581]; P = 0.001) was predictive for a higher risk of developing fine motor abnormalities, higher postmenstrual age (aOR = 0.701; 95% CI [0.482–1.020]; P = 0.04) was predictive for a lower risk of developing fine motor abnormalities at preschool age. Group assignment, cumulative opiate exposure, occurrence of NEC requiring surgery, and BPD had no impact on gross motor and fine motor skills at preschool age (Table 3).
Firth logistic regression analysis of risk factors for the prediction of parent-reported CBCL scores is provided in Table 4. Every increase of 1 mg/kg cumulative opiate exposure was weakly significantly associated with autism spectrum features (aOR = 1.006, 95% CI [1.002–1.010]; P = 0.03) and withdrawn behavior (aOR = 1.006, 95% CI [1.002–1.010]; P = 0.03) in the CBCL at preschool age. For infants who received a cumulative dose of 100 mg/kg, the odds for both exhibiting autism spectrum features (aOR = 1.822, 95% CI [1.231–2.697]; P = 0.03) and withdrawn behavior (aOR = 1.822, 95% CI [1.231–2.697]; P = 0.03) in the CBCL at preschool age was 1.82 times higher, when compared to infants who did not receive any opiates in the neonatal period (Table 4).
Discussion
In this single-center observational study of extremely preterm infants managed according to V-PNPS,11 we did not observe any long-term detriments on cognition, motor function, and growth parameters, as well as hearing and visual function compared to a historical control group, even though implementation of the protocol resulted in significantly higher cumulative opiate exposure. Nevertheless, we found that increased cumulative opiate exposure may represent a potential risk factor for developing autism spectrum features and withdrawn behavior in the parent-reported CBCL at the preschool follow-up examination.
Cognitive outcomes
The sequential processing scale is an important subscale of the KABC measuring short-term and working memory. Our finding that the higher cumulative opiate exposure associated with implementation of the V-PNPS did not have an impact on sequential processing scale scores was also confirmed by de Graaf and colleagues.23 They did not find any difference between morphine and control group infants in working memory at the age of 8 and 9 years.23 Conversely, Ferguson and colleagues24 showed that morphine-exposed infants exhibited significantly longer choice response latencies indicating an impaired short-term memory when compared to children treated with placebo at 5–7 years of age.
We also used the simultaneous processing scale, a subscale part of the KABC measuring visual memory, spatial relations, and visual motor integration. Poorer visual motor integration is more common in preterm infants when compared to full-term infants.25 However, neonatal morphine exposure was not associated with poorer visual motor integration scores when compared to placebo-treated infants at the age of 526 and 8–9 years.23 These findings are consistent with our results. Increased cumulative opiate exposure was not a risk factor for lower scores in the simultaneous processing scale at preschool age. Similar results were also found in our 1-year12 and 3-year follow-up.13 In both studies, the mental developmental index, a subscale of the Bayley Scales of Infant Development measuring cognitive function, was unaffected by the increased cumulative opiate exposure.
Motor outcomes
The implementation of the V-PNPS and the associated increased cumulative opiate exposure did not have an impact on motor outcomes in the intervention group neither when comparing the incidence of motor abnormalities between the groups nor when looking at the logistic regression model considering cumulative opiate exposure as a risk factor for the prediction of motor abnormalities. Only a few studies have investigated the association of postnatal morphine exposure on motor outcomes after a follow-up of several years, and results are contradictory. Results of the present study are in line with our previous follow-up studies. At 1-year12 and 3-year13 follow-up, increased cumulative opiate exposure was not associated with altered motor development. In addition, our data are consistent with results by MacGregor and colleagues.27 A study by Ferguson and colleagues24 assessed motor outcome of a small number of children who participated in the NEOPAIN (Neurologic Outcomes and Pre-emptive Analgesia in Neonates) trial at the age of 5–7 years and found no significant difference in motor function between the morphine (n = 14) and placebo (n = 5) groups. However, definite conclusions cannot be drawn from this small sample size study. Conversely, Grunau and colleagues reported that greater intravenous morphine exposure was associated with poorer motor outcomes at the corrected age of 8 months but not at 18 months.28 However, there was no specific morphine protocol and morphine was administered by clinical judgement only.
Behavioral outcomes
Autism spectrum disorders (ASD)
There is growing evidence that a combination of perinatal risk factors including preterm birth,29 small for gestational age,30 and perinatal hypoxia31 contribute to an increased risk of developing ASD. However, the impact of these risk factors and the role of morphine for the development of ASD is still unclear.
In our study, cumulative opiate exposure seemed to be a risk factor for exhibiting abnormal autism spectrum problem scores in the parent-reported CBCL. Multiple lines of evidence from animal models suggested an association of postnatal morphine exposure with Purkinje cell death32 and altered cerebellar development.33 Moreover, it has been reported that neonatal morphine exposure is also associated with impaired cerebellar growth in the human neonate, independently of additional prominent perinatal risk factors.34
Interestingly, alterations in the cerebellum and Purkinje cell function have been associated with a higher incidence of ASD in preterm infants35 and behavioral deficits relevant to ASD in an animal model.36 Thus there might be an association between morphine-induced alteration of cerebellar growth and development of ASD. However, to date no study has examined the link between morphine-induced brain alterations and development of ASD in preterm infants.
Recently, Chau and colleagues showed that higher neonatal morphine exposure was associated with more internalizing and externalizing behaviors at the corrected age of 18 months in infants born very preterm. Interestingly, these effects were moderated by genetic markers in the morphine metabolic pathway including UDP-glucuronosyltransferase and catechol-O-methyltransferase. In addition, they found a dose-dependent association of morphine exposure on CBCL scores at the corrected age of 18 months after adjusting for other clinical confounders. Thus higher dosing of morphine was associated with a clinically important impact on behavior.37 Although we did not include genetic variants as cofactors in our regression analysis, this is in line with our study as we also found a dose-dependent effect of neonatal morphine exposure on behavior.
Withdrawn behavior
Preterm birth is associated with a higher risk of developing internalizing behaviors including withdrawn and anxious/depressed behavior, as well as somatic problems when compared with infants born at term.38 Multivariable linear regression analysis of potential risk factors showed that cumulative opiate exposure was associated with an increased risk of developing clinical apparent withdrawn scores in the parent-reported CBCL in our study. These findings are confirmed by animal models and human studies. Evidence from animal models suggests that neonatal morphine exposure might result in depression-like behavior in juvenile and adult rats.39 This is in line with a study by Ranger and colleagues40 who identified higher neonatal morphine exposure in infants born very preterm as a risk factor for developing higher internalizing behavior scores in the parent-reported CBCL.
Limitations
This study was initially designed to assess the implementation of a protocol for the management of pain and sedation11 and provides a unique opportunity to investigate the impact of opiates on outcomes at preschool age. Although the parent-reported CBCL is not a diagnostic tool for making the final diagnosis of ASD, high accuracy was reported to distinguish preschool infants with an ASD from preschool infants with a normal development.41 Consequently, the CBCL is a good screening tool but does not replace ASD-specific diagnostic instruments. Moreover, another weakness of the study was the drop-out rate throughout the whole follow-up period. However, our two study groups were well matched. Even if there were no significant differences between the two groups at baseline, we were not able to completely rule out any other confounding factors. Consequently, we tried to control for the most prominent risk factors, but as in every clinical study, it was not possible to consider our patients within their complete environment. For this reason, to contextualize our P values, we always provided estimates, ORs, and CIs. Given the exploratory nature of this study and the influence of potential unknown confounding factors, P values should be interpreted with caution and not allow any conclusions on causality.
Conclusion
The implementation of a protocol for the management of pain and sedation in preterm infants resulted in increased cumulative opiate exposure but did not impair cognitive and motor outcomes at the age of 5–6 years. However, we found that higher cumulative opiate exposure may represent a possible risk factor for developing autism spectrum features and withdrawn behavior in the parent-reported CBCL at preschool age. Thus a judicious use of opiates is recommended, and new studies should be conducted searching for new pain management strategies and drugs providing optimal pain relief with minimal neurotoxicity.
References
Carbajal, R. et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA 300, 60–70 (2008).
Smith, G. C. et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann. Neurol. 70, 541–549 (2011).
Brummelte, S. et al. Procedural pain and brain development in premature newborns. Ann. Neurol. 71, 385–396 (2012).
Vinall, J., Miller, S. P., Synnes, A. R. & Grunau, R. E. Parent behaviors moderate the relationship between neonatal pain and internalizing behaviors at 18 months corrected age in children born very prematurely. Pain 154, 1831–1839 (2013).
Taddio, A., Shah, V., Gilbert-MacLeod, C. & Katz, J. Conditioning and hyperalgesia in newborns exposed to repeated heel lances. JAMA 288, 857–861 (2002).
Bajic, D., Commons, K. G. & Soriano, S. G. Morphine-enhanced apoptosis in selective brain regions of neonatal rats. Int. J. Dev. Neurosci. 31, 258–266 (2013).
Handelmann, G. E. & Dow-Edwards, D. Modulation of brain development by morphine: effects on central motor systems and behavior. Peptides 6(Suppl 2), 29–34 (1985).
Anand, K. J. et al. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet 363, 1673–1682 (2004).
Simons, S. H. et al. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA 290, 2419–2427 (2003).
Anand, K. J. Consensus statement for the prevention and management of pain in the newborn. Arch. Pediatr. Adolesc. Med. 155, 173–180 (2001).
Deindl, P. et al. Successful implementation of a neonatal pain and sedation protocol at 2 NICUs. Pediatrics 132, e211–e218 (2013).
Deindl, P. et al. The implementation of systematic pain and sedation management has no impact on outcome in extremely preterm infants. Acta Paediatr. 105, 798–805 (2016).
Giordano, V. et al. Effect of increased opiate exposure on three years neurodevelopmental outcome in extremely preterm infants. Early Hum. Dev. 123, 1–5 (2018).
Gammaitoni, A. R., Fine, P., Alvarez, N., McPherson, M. L. & Bergmark, S. Clinical application of opioid equianalgesic data. Clin. J. Pain 19, 286–297 (2003).
Gosselin, J., Gahagan, S. & Amiel-Tison, C. The Amiel-Tison neurological assessment at term: conceptual and methodological continuity in the course of follow-up. Ment. Retard. Dev. Disabil. Res. Rev. 11, 34–51 (2005).
Melchers, P. & Preuß, U. Kaufman Assessment Battery for Children (deutsche Version) (8., unveränd. Aufl.) (Pearson Assessment, Frankfurt/M, 2009).
Achenbach, T. & Rescorla, L. Manual for the ASEBA Preschool Forms & Profiles (University of Vermont, Research Center for Children, Youth, & Families, Burlington, VT, 2000).
Kaufman, A. S. & Kaufman, N. L. KABC-II. Kaufman Assessment Battery for Children- II. Deutschsprachige Fassung von P. Melchers und M. Melchers (Pearson, Frankfurt am Main, 2015).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (Washington, DC, 2013).
King, G. & Zeng, L. Logistic regression in rare events data. Political Anal. 9, 137–163 (2001).
Faul, F., Erdfelder, E., Buchner, A. & Lang, A. G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods 41, 1149–1160 (2009).
Vohr, B. R. et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993-1994. Pediatrics 105, 1216–1226 (2000).
de Graaf, J. et al. Does neonatal morphine use affect neuropsychological outcomes at 8 to 9 years of age? Pain 154, 449–458 (2013).
Ferguson, S. A., Ward, W. L., Paule, M. G., Hall, R. W. & Anand, K. J. A pilot study of preemptive morphine analgesia in preterm neonates: effects on head circumference, social behavior, and response latencies in early childhood. Neurotoxicol. Teratol. 34, 47–55 (2012).
Bolk, J. et al. National population-based cohort study found that visual-motor integration was commonly affected in extremely preterm born children at six-and-a-half years. Acta Paediatr. 107, 831–837 (2018).
de Graaf, J. et al. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children’s functioning: five-year follow-up of a randomized controlled trial. Pain 152, 1391–1397 (2011).
MacGregor, R., Evans, D., Sugden, D., Gaussen, T. & Levene, M. Outcome at 5-6 years of prematurely born children who received morphine as neonates. Arch. Dis. Child. Fetal Neonatal Ed. 79, F40–F43 (1998).
Grunau, R. E. et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain 143, 138–146 (2009).
Schendel, D. & Bhasin, T. K. Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics 121, 1155–1164 (2008).
Moore, G. S., Kneitel, A. W., Walker, C. K., Gilbert, W. M. & Xing, G. Autism risk in small- and large-for-gestational-age infants. Am. J. Obstet. Gynecol. 206, 314.e311–319 (2012).
Burstyn, I., Wang, X., Yasui, Y., Sithole, F. & Zwaigenbaum, L. Autism spectrum disorders and fetal hypoxia in a population-based cohort: accounting for missing exposures via Estimation-Maximization algorithm. BMC Med. Res. Methodol. 11, 2 (2011).
Hauser, K. F., Gurwell, J. A. & Turbek, C. S. Morphine inhibits Purkinje cell survival and dendritic differentiation in organotypic cultures of the mouse cerebellum. Exp. Neurol. 130, 95–105 (1994).
Bekheet, S. H., Saker, S. A., Abdel-Kader, A. M. & Younis, A. E. Histopathological and biochemical changes of morphine sulphate administration on the cerebellum of albino rats. Tissue Cell 42, 165–175 (2010).
Zwicker, J. G. et al. Smaller cerebellar growth and poorer neurodevelopmental outcomes in very preterm infants exposed to neonatal morphine. J. Pediatr. 172, 81–87.e82 (2016).
Limperopoulos, C. et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 120, 584–593 (2007).
Tsai, P. T. et al. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 488, 647–651 (2012).
Chau, C. M. Y. et al. Morphine biotransformation genes and neonatal clinical factors predicted behaviour problems in very preterm children at 18 months. EBioMedicine 40, 655–662 (2019).
Aarnoudse-Moens, C. S., Weisglas-Kuperus, N., van Goudoever, J. B. & Oosterlaan, J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 124, 717–728 (2009).
Klausz, B. et al. Changes in adaptability following perinatal morphine exposure in juvenile and adult rats. Eur. J. Pharmacol. 654, 166–172 (2011).
Ranger, M., Synnes, A. R., Vinall, J. & Grunau, R. E. Internalizing behaviours in school-age children born very preterm are predicted by neonatal pain and morphine exposure. Eur. J. Pain. 18, 844–852 (2014).
Narzisi, A. et al. Child Behavior Check List 1(1/2)-5 as a tool to identify toddlers with autism spectrum disorders: a case-control study. Res. Dev. Disabil. 34, 1179–1189 (2013).
Acknowledgements
We gratefully acknowledge all medical doctors, nurses, and psychologists at the Division of Neonatology, Pediatric Intensive Care and Neuropediatrics at the Medical University of Vienna for performing follow-up examinations and thank the families for their participation.
Author information
Authors and Affiliations
Contributions
All authors conceptualized the study and drafted the initial manuscript. The data were analyzed by P.S., V.G., P.D., FC, R.F., L.U., and M.O. The manuscript was drafted and critically reviewed and revised by all authors. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Written informed consent was obtained from the parents of the patients prior to inclusion. The study has been approved by our local institutional review board.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Steinbauer, P., Deindl, P., Fuiko, R. et al. Long-term impact of systematic pain and sedation management on cognitive, motor, and behavioral outcomes of extremely preterm infants at preschool age. Pediatr Res 89, 540–548 (2021). https://doi.org/10.1038/s41390-020-0979-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0979-2
This article is cited by
-
Early-life exposure to analgesia and 18-month neurodevelopmental outcomes in very preterm infants
Pediatric Research (2023)
-
RETRACTED ARTICLE: The Neonatal Withdrawal Assessment Tool (NWAT): pilot inter-rater reliability and content validity
Journal of Perinatology (2023)
-
Neonatal opioids and preschool outcomes
Pediatric Research (2021)