Abstract
Background
To evaluate the apoptosis inhibitor of macrophage (AIM) deposition patterns on the kidneys of children with IgA nephropathy (IgAN) and Henoch–Schönlein purpura nephritis (HSPN) and to investigate the clinical usefulness of serum and/or urinary AIM levels as biomarkers for the disease activity.
Methods
Immunohistochemical study was performed in the kidneys of 37 patients with IgAN and 10 patients with HSPN. Serum and urinary AIM levels in the patients and 20 healthy controls (HCs) were quantified by enzyme-linked immunosorbent assay. The results were compared with clinical features.
Results
In patients with IgAN and HSPN, AIM expression was observed in various areas, including the glomerular mesangial and capillary areas, the proximal and distal tubular epithelial cells, and on infiltrating macrophages in the glomeruli and interstitial areas. Serum and urinary AIM levels were significantly elevated in these patients compared with the HCs. Urinary AIM levels were positively correlated with the histological severity and degree of proteinuria and hematuria as well as urinary β2 microglobulin and urinary N-acetyl-β-D-glucosaminidase levels.
Conclusions
AIM plays an important role in the pathogenesis of IgAN and HSPN. Urinary AIM levels can potentially reflect active renal inflammation in these diseases and may represent a useful biomarker for disease activity.
Impact
-
Urinary AIM levels may represent a useful biomarker for disease activity of IgAN and HSPN.
-
AIM expression was observed in the glomeruli, tubular epithelial cells, and infiltrating macrophages in glomeruli and interstitial area.
-
U-AIM/Cr were significantly correlated not only with proteinuria, hematuria, and u-β2MG and u-NAG levels but also with the activity index of histological findings in kidney biopsy specimens.
-
Our results can emphasize the important role of AIM in the pathogenesis of IgAN and HSPN.
Similar content being viewed by others
Introduction
Apoptosis inhibitor of macrophage (AIM), also known as CD5-like (CD5L), is a secreted protein of the scavenger receptor cysteine-rich superfamily that enables macrophage survival from a variety of apoptosis-inducing agents.1 AIM has been implicated in a variety of inflammatory diseases, including atherosclerosis and metabolic syndrome.2,3,4 For instance, upregulated AIM has been found to suppress foam cell apoptosis and hence promote hyperlipidemic atherosclerosis.2 AIM has also been associated with the progression of metabolic syndrome progression, including the development of obesity and insulin resistance.3,4 Furthermore, recent accumulating evidence has revealed that AIM plays an important role in the pathogenesis of hepatic fibrosis,5 chronic obstructive pulmonary disease,6 inflammatory bowel disease,7 and rheumatic diseases,8,9 by protecting macrophages from apoptosis and thus resulting in prolonged inflammation. Besides macrophages, AIM is also known to be associated with the proliferation of B and T helper type 17 cells.10,11 Therefore, and based on existing research, AIM may play a critical role in immune homeostasis and inflammatory disease control.
When it comes to the kidneys, AIM plays an important role in acute kidney injury (AKI) because the majority of circulating AIM proteins are bound to immunoglobulin M (IgM) via Fc region recovery.12 In fact, this binding to IgM prevents excretion of AIM in urine, thereby sustaining the respective circulating AIM concentrations. In case of AKI, AIM is excreted in urine and accumulates on necrotic cell debris within the kidney proximal tubule. However, the exact mechanism with which AKI facilitates the dissociation of AIM from IgM in the blood is still unknown.12 Nevertheless, AIM in the cellular debris binds to kidney injury molecule-1 that is expressed on injured tubular epithelial cells, which enhances the phagocytic removal of the debris by the epithelial cells and then contributes to kidney tissue repair.12
Kidney biopsy is essential for assessing the degree of inflammation and tubulointerstitial damage in chronic kidney disease. However, biopsy is invasive and cannot be repeated frequently to evaluate therapeutic responses, especially in children. Therefore, it is necessary to develop novel, non-invasive biomarkers for evaluating the degree and the characteristics of inflammation and tubulointerstitial damage.
The aim of this study was to evaluate the patterns of AIM expression in kidneys in children with IgA nephropathy (IgAN) and Henoch–Schönlein purpura nephritis (HSPN), which are the most common types of glomerulonephritis in children. Furthermore, this study intended to clarify whether serum and/or urinary AIM levels can be useful biomarkers for disease activity of IgAN and HSPN.
Methods
Patients and samples
In this study, we enrolled 37 patients with IgAN and 10 patients with HSPN. Serum and urine samples were obtained from 47 patients and 20 age- and sex-matched healthy controls (HCs). All samples were collected at the time of kidney biopsy. This study was approved by the Institutional Review Board of Kanazawa University, Japan, and written informed consents were obtained from all participants.
Urinalysis
Urinalysis and urinary biochemical studies were performed with fresh urine specimens. The residual samples were stored at −80 °C for further analysis. Hematuria was assessed by the number of red blood cells per high-power field in a fresh urine sample. Furthermore, proteinuria was measured by the urinary protein/creatinine ratio based on the first morning urine sample.
Renal tissue processing
Kidney tissue samples used for light microscopy and immunohistochemical investigations were fixed in 4% buffered paraformaldehyde that was dehydrated with graded concentrations of ethanol and xylene and then embedded in paraffin. Four-μm-thick frozen sections were fixed in cold acetone and stored at −20 °C for the required routine immunofluorescence studies. All specimens were investigated for IgG, IgA, and IgM deposits, complement (C)1q, C3, C4, and fibrinogen using direct immunofluorescence. The histological activity and chronicity scores of IgAN and HSPN were extracted from a scoring system of specific histological features.13 The activity index was assessed based on the percentage of glomeruli with cellular crescents (score 0, 0%; 1, 1–20%; 2, 20–50%, 3, >50%), cellular proliferation (score 0–3), necrosis (score 0, absent; 1, present), and interstitial mononuclear cell infiltration (score 0–3). Moreover, the chronicity index was assessed based on the percentage of glomeruli with fibrous crescent formation (score 0, 0%; 1, 1–20%; 2, 20–50%, 3, >50%) and segmental and global sclerosis (score 0–3) and atrophic tubule and/or interstitial fibrosis (score 0–3). The sum of these scores presented the activity and chronicity indices.
Immunohistochemistry
Immunostaining for AIM or macrophage-specific antigen CD68 was performed with an anti-AIM rabbit antiserum (Abcam, Cambridge, UK) or an anti-CD68 monoclonal antibody (Dako Co., Carpinteria, CA). Paraffin sections of the kidney were deparaffinized and rehydrated with descending ethanol. The sections were immersed in Target-Retrieval Solution (Dako) and were heated in a microwave oven for 5 min at 500 W. The slides were then immersed in hot Target-Retrieval Solution to cool for 30 min. After rinsing in Tris buffer, tissue sections were blocked with 4% of normal goat serum and incubated with appropriate dilutions of anti-AIM or anti-CD68 antibody for 12 h at 4 °C. After rinsing in Tris buffer, the sections were incubated with EnVision polymer reagents (Dako), dextran polymer conjugated with alkaline phosphatase, and goat anti-rabbit/mouse IgG for 30 min at room temperature. Finally, alkaline phosphatase activity was then visualized using Fast Red TR salt (Sigma Chemical Co., St. Louis, MO).
Quantitative evaluation of AIM protein levels
The level of AIM expression within the glomeruli and the tubule were evaluated separately. The intensity and the distribution of AIM expression within tubule were evaluated as we previously reported.14,15 AIM expression within the tubule was graded into 0 to 3 as follows: 0, no staining; 1, weak and focal; 2, weak and diffuse or strong and focal; 3, strong and diffuse (Supplementary Fig. S1). The level of AIM expression within glomeruli were evaluated by the patterns of its distribution. The levels were graded into 0 to 3 as follows: 0, no staining; 1, focal and segmental; 2, diffuse and segmental or focal and global; 3, diffuse and global (Supplementary Fig. S1). The level of AIM protein staining was graded from 0 to 4 as follows: 0, no staining; 1, weak and focal; 2, weak and diffuse or moderate and focal; 3, moderate and diffuse or strong and focal; 4, strong and diffuse. Five nephrologists examined the specimens independently and assessed the intensity and the distribution without knowledge of the patient’s histological diagnosis.
Enzyme-linked immunosorbent assay for detecting AIM levels
Serum and urinary AIM levels were assessed by enzyme-linked immunosorbent assay according to the manufacturer’s instructions (Circulex Human AIM/CD5L/Spα ELISA Kit, MBL, Nagoya, Japan).
Statistical analysis
All data were expressed as median with interquartile range (IQR). Comparisons among three groups were performed using one-way analysis of variance with Tukey’s multiple comparison test. Furthermore, Spearman’s rank correlation coefficient was used to express the respective correlations. A P value of <0.05 was considered to be statistically significant.
Results
Clinical characteristics
The clinical characteristics of the 37 patients with IgAN and 10 patients with HSPN are summarized in Table 1.
Expression patterns of AIM on kidney in IgAN and HSPN
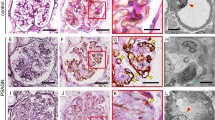
AIM expression was observed in various areas, including the glomerular mesangial (Fig. 1a) and capillary (Fig. 1a), as well as in the proximal and distal tubular epithelial cells (Fig. 1c), in patients with IgAN and HSPN. AIM expression was also observed on infiltrating macrophages in the glomeruli and the interstitial area (Fig. 1b, d–f).
AIM expression was observed in various areas, including the glomerular mesangial and capillary areas (a; original magnification ×200) as well as in the proximal and distal tubular epithelial cells (c; original magnification ×100) in patients with IgA nephropathy and Henoch–Schönlein purpura nephritis. Immunohistochemical examinations of the consecutive kidney sections indicated that AIM-staining cells in the glomeruli (b; original magnification ×200) and interstitium (e; original magnification ×400) were both CD68-positive macrophages (d; original magnification ×200, f; original magnification ×400).
Increased serum and urinary AIM levels in children with IgAN and HSPN
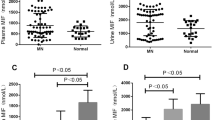
As shown in Fig. 2a, serum AIM levels were significantly elevated in children with IgAN (median, 854.9 ng/ml, IQR, 635.4–1175.4 ng/mL; P < 0.0001) and HSPN (721.4, 592.9–1164.7; P < 0.05) compared to the HCs (429.2, 326.2–527.5). Furthermore, Fig. 2b demonstrates that the urinary AIM levels/creatinine ratio (U-AIM/Cr) were also significantly elevated in children with IgAN (median, 1.217 μg/gCr, IQR, 0.846–2.047 μg/gCr; P < 0.0001) and HSPN (2.646, 0.855–4.349; P < 0.0001) compared to the HCs (0.370, 0.304–0.548). There were no differences in serum AIM levels and U-AIM/Cr ratio between IgAN and HSPN. Finally, there was no correlation between serum AIM levels and U-AIM/Cr in children with IgAN (Fig. 1c) and HSPN (Fig. 1d).
Serum (a) and urinary (b) levels of AIM in children with IgA nephropathy and Henoch–Schönlein purpura nephritis are shown. The correlation between serum and urinary levels of AIM in children with IgA nephropathy is shown in c. The correlation between serum and urinary levels of AIM in children with Henoch–Schönlein purpura nephritis is shown in d. Values are displayed as dot plots. Bars represent mean ± SD. HC healthy control, IgAN IgA nephropathy, HSPN Henoch–Schönlein purpura nephritis, S-AIM serum AIM levels, U-AIM/Cr urinary AIM/creatinine ratio, P P value.
Relationship between serum/urinary AIM levels and histological findings in IgAN and HSPN
Serum AIM levels were not correlated with the degree of glomerular AIM expression in children with IgAN and HSPN (Supplementary Fig. 2A, B). Serum AIM levels were not also correlated with the degree of tubular AIM expression in children with IgAN (Supplementary Fig. 2C), although serum AIM levels were correlated with the degree of tubular AIM expression in children with HSPN (Supplementary Fig. 2D). U-AIM/Cr ratio did not demonstrate any correlation with the degree of glomerular and tubular AIM expression in children with IgAN and HSPN (Supplementary Fig. 2E–H).
Serum AIM levels were not correlated with the histological severity, including the activity (Fig. 3a, b) and chronicity indices (Fig. 3c, d) in children with IgAN and HSPN. U-AIM/Cr ratios were significantly and positively correlated with the activity index (Fig. 3e, f) in children with IgAN and HSPN but not correlated with the chronicity index (Fig. 3g, h).
Correlation between serum AIM levels and activity index (a, b) and chronicity index (c, d) are shown. Correlation between urinary AIM levels and activity index (e, f) and chronicity index (g, h) are shown. a, c, e, g; IgA nephropathy, b, d, f, h; Henoch–Schönlein purpura nephritis. AI activity index, CI chronicity index, S-AIM serum AIM levels, U-AIM/Cr urinary AIM/creatinine ratio.
Relationship between serum/urinary AIM levels and clinical parameters in IgAN and PN
Serum AIM levels were not correlated with any clinical parameters in children with IgAN other than serum IgA levels, including proteinuria, hematuria, serum Cr levels, estimated glomerular filtration rate (eGFR), urinary β2 microglobulin (u-β2MG), and urinary N-acetyl-β-D-glucosaminidase (u-NAG) levels. Serum AIM levels were not correlated with any clinical parameters in children with HSPN. In contrast, U-AIM/Cr was significantly and positively correlated with proteinuria (Fig. 4a), hematuria (Fig. 4b), and u-β2MG (Fig. 4c) and u-NAG levels (Fig. 4d) in children with IgAN. U-AIM/Cr was not correlated with proteinuria (Fig. 4e), hematuria (Fig. 4f), and u-β2MG (Fig. 4g) but was significantly and positively correlated with u-NAG levels (Fig. 4h) in children with HSPN. U-AIM/Cr was not correlated with eGFR in children with IgAN and PN (data not shown).
Correlation between urinary AIM levels and proteinuria (a), hematuria (b), and urinary β2 microglobulin (c) and urinary N-acetyl-β-D-glucosaminidase levels (d) in patients with IgA nephropathy are shown. Correlation between urinary AIM levels and proteinuria (e), hematuria (f), and urinary β2 microglobulin (g) and urinary N-acetyl-β-D-glucosaminidase levels (h) in patients with Henoch–Schönlein purpura nephritis are shown (U-Pro/Cr urinary protein/creatinine ratio, U-AIM/Cr urinary AIM/creatinine ratio, RBC red blood cells, Hpf high-power field, u-β2MG/Cr urinary β2 microglobulin/creatinine ratio, u-NAG/Cr urinary N-acetyl-β-D-glucosaminidase/creatinine ratio).
Discussion
In this study, we showed that AIM expression in patients with IgAN and HSPN was observed in various areas, including the glomerular mesangial the capillary areas, in the proximal and distal tubular epithelial cells, and in the infiltrating macrophages in glomeruli and interstitial area. Furthermore, we demonstrated that serum AIM levels and U-AIM/Cr were significantly elevated in patients with IgAN and HSPN compared to the HCs. Serum AIM levels were not correlated with clinical parameters and histological severity in patients with IgAN and HSPN. In contrast, U-AIM/Cr was significantly correlated not only with proteinuria, hematuria, and u-β2MG and u-NAG levels but also with the activity index of histological findings in kidney biopsy specimens in patients with IgAN. Furthermore, U-AIM/Cr was significantly correlated with not only u-NAG levels but also with the activity index of histological findings in kidney biopsy specimens in patients with HSPN. These findings indicate that AIM plays an important role in the pathogenesis of IgAN and HSPN, and U-AIM/Cr might be a useful biomarker for these diseases.
Macrophages play important roles in the pathogenesis of immune complex-mediated glomerulonephritis. Glomerular macrophage accumulation is often observed, indicating disease activity or/and severity. Macrophages remove the immune complexes deposited in the subendothelial and/or mesangial area of the glomeruli in IgAN and HSPN.16 Furthermore, macrophages produce various cytotoxic products, such as proinflammatory cytokines, and chemokines, that may facilitate mesangial cell proliferation and tubular cell apoptosis.17,18,19 In this study, immunohistochemical staining revealed that AIM expression was observed in various areas, such as the mesangial and capillary areas of the glomeruli. Furthermore, AIM was positively identified in CD68-positive infiltrating macrophages. These results are in agreement with Ohshima et al.20 One of the functions of AIM is to prevent macrophage apoptosis, thus suggesting that AIM may be contributing to kidney injury by prolonging local inflammation, at least partly. On the other hand, AIM contributes to kidney tissue repair by enhancing the phagocytic removal of the debris facilitated by the epithelial cells.12 In this study, AIM expression was also observed in tubule. These findings indicate that AIM might play an important role in the kidney injury recovery from glomerulonephritis, at least partly. Although the role of AIM in the pathogenesis of IgAN and HSPN is still unclear, AIM might have multilateral functions in the kidney.
Elevation of serum AIM levels has been reported in several inflammatory diseases.5 For instance, observed increased serum levels of AIM in patients with chronic hepatitis C patients were potentially related to the development of advanced hepatic fibrosis.5 Furthermore, observed increased serum levels of AIM in patients with systemic lupus erythematosus (SLE) were associated with the disease activity.9 In addition, the ex vivo release of AIM upon mitogen activation of peripheral blood mononuclear cells was significantly increased in SLE patients compared to the HCs.9 Subsequently, aberrant production of AIM may be closely associated with the development of inflammatory diseases. In this study, serum AIM levels were significantly elevated in patients with IgAN and HSPN compared to the HCs. These findings may indicate aberrant production of AIM based on immune dysregulations and can also be closely associated with the development of IgAN and HSPN. Further studies are needed to evaluate the ex vivo release of AIM upon mitogen activation of immune cells in patients with IgAN and HSPN.
In this study, serum AIM levels were not correlated with the histological severity and clinical parameters in order to reflect disease activity in IgAN and HSPN. In contrast, U-AIM/Cr was significantly and positively correlated with the histological activity of IgAN and HSPN and with clinical parameters including proteinuria, hematuria, and u-β2MG and u-NAG levels in patients with IgAN. Furthermore, U-AIM/Cr was significantly correlated not only with u-NAG levels but also with the activity index of histological findings in kidney biopsy specimens in patients with HSPN. These findings suggest that U-AIM/Cr may represent a promising indicator of disease activity in patients with IgAN and HSPN. A previous study using ischemia–reperfusion-induced AKI model mice revealed that free AIM in the serum appears to be the major source of urinary AIM.12 In this study, there was no correlation found between the serum AIM levels and U-AIM/Cr. Furthermore, there was no correlation identified between U-AIM/Cr and the degree of glomerular and tubular AIM deposition. Consequently, these results underline that free AIM in the serum did not appear to be the only major source of urinary AIM in children with IgAN and HSPN. It is well known that macrophages are the major cellular source of AIM.1 In this study, AIM deposition was observed in infiltrating macrophages in both the glomeruli and the tubulointerstitial area. It is well known that infiltrating macrophages can cause injury in immune-mediated glomerulonephritis. Therefore, it can be suggested that infiltrating macrophages may be considered to be an additional cellular source of urinary AIM.
The limitation of this study is the small sample size of patients with IgAN and HSPN employed. Hence, it is necessary to perform larger-scale studies to confirm our preliminary findings and verify our conclusions.
Conclusions
AIM expression was observed in various areas, including the glomerular mesangial and capillary areas, the proximal and distal tubular epithelial cells, and the infiltrating macrophages in glomeruli and interstitial areas of patients with IgAN and HSPN. These results indicate that AIM plays an important role in the pathogenesis of these diseases. Furthermore, U-AIM/Cr were significantly correlated with clinical parameters to reflect disease activity as well as an activity index of histological findings in kidney biopsy specimen. These findings indicate that increased urinary AIM levels may reflect active kidney inflammation in IgAN and HSPN. Therefore, AIM could be considered as a useful biomarker for disease activity of these diseases in clinical settings.
References
Miyazaki, T., Hirokami, Y., Matsuhashi, N., Takatsuka, H. & Naito, M. Increased susceptibility of thymocytes to apoptosis in mice lacking AIM, a novel murine macrophage-derived soluble factor belonging to the scavenger receptor cysteine-rich domain superfamily. J. Exp. Med. 189, 413–422 (1999).
Hamada, M. et al. MafB promotes atherosclerosis by inhibiting foam-cell apoptosis. Nat. Commun. 5, 3147 (2014).
Miyazaki, T., Kurokawa, J. & Arai, S. AIMing at metabolic syndrome. Towards the development of novel therapies for metabolic disease via apoptosis inhibitor of macrophage (AIM). Circ. J. 75, 2522–2531 (2011).
Arai, S. & Miyazaki, T. Impacts of the apoptosis inhibitor of macrophage (AIM) on obesity-associated inflammatory diseases. Semin. Immunopathol. 36, 3–12 (2014).
Mera, K. et al. Serum levels of apoptosis inhibitor of macrophage are associated with hepatic fibrosis in patients with chronic hepatitis C. BMC Gastroenterol. 14, 27 (2014).
Kojima, J. et al. Apoptosis inhibitor of macrophage (AIM) expression in alveolar macrophages in COPD. Respir. Res. 14, 30 (2013).
Haruta, I. et al. Apoptosis inhibitor expressed by macrophages tempers autoimmune colitis and the risk of colitis-based carcinogenesis in TCRalpha−/− mice. J. Clin. Immunol. 27, 549–556 (2007).
Balakrishnan, L. et al. Differential proteomic analysis of synovial fluid from rheumatoid arthritis and osteoarthritis patients. Clin. Proteomics 11, 1 (2014).
Lai, X., Xiang, Y., Zou, L., Li, Y. & Zhang, L. Elevation of serum CD5L concentration is correlated with disease activity in patients with systemic lupus erythematosus. Int. Immunopharmacol. 63, 311–316 (2018).
Yusa, S., Ohnishi, S., Onodera, T. & Miyazaki, T. AIM, a murine apoptosis inhibitory factor, induces strong and sustained growth inhibition of B lymphocytes in combination with TGF-beta1. Eur. J. Immunol. 29, 1086–1093 (1999).
Wang, C. et al. CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell pathogenicity. Cell 163, 1413–1427 (2015).
Arai, S. et al. Apoptosis inhibitor of macrophage protein enhances intraluminal debris clearance and ameliorates acute kidney injury in mice. Nat. Med. 22, 183–193 (2016).
Andreoli, S. P., Yum, M. N. & Bergstein, J. M. IgA nephropathy in children: significance of glomerular basement membrane deposition of IgA. Am. J. Nephrol. 6, 28–33 (1986).
Morimoto, K. et al. Cytoprotective role of heme oxygenase (HO)-1 in human kidney with various renal diseases. Kidney Int. 60, 1858–1866 (2001).
Shimizu, M. et al. Glomerular proteinuria induces heme oxygenase-1 gene expression within renal epithelial cells. Pediatr. Res. 58, 666–671 (2005).
Striker, G. E., Mannik, M. & Tung, M. Y. Role of marrow-derived monocytes and mesangial cells in removal of immune complexes from renal glomeruli. J. Exp. Med. 149, 127–136 (1979).
Nathan, C. F., Murray, H. W. & Cohn, Z. A. The macrophage as an effector cell. N. Engl. J. Med. 303, 622–626 (1980).
Ricardo, S. D., van Goor, H. & Eddy, A. A. Macrophage diversity in renal injury and repair. J. Clin. Invest. 118, 3522–3530 (2008).
Lange-Sperandio, B., Fulda, S., Vandewalle, A. & Chevalier, R. L. Macrophages induce apoptosis in proximal tubule cells. Pediatr. Nephrol. 18, 335–341 (2003).
Oshima, M. et al. Association of apoptosis inhibitor of macrophage (AIM) expression with urinary protein and kidney dysfunction. Clin. Exp. Nephrol. 21, 35–42 (2017).
Author information
Authors and Affiliations
Contributions
H.I., M.S., S.K., N.I., M.M., Y.T., and K.O. were involved in the conception, design of the study, and the acquisition of data. H.I. and M.S. were involved in analysis, interpretation of data, and drafting of the manuscript. A.Y. and T.W. revised it critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Irabu, H., Shimizu, M., Kaneko, S. et al. Apoptosis inhibitor of macrophage as a biomarker for disease activity in Japanese children with IgA nephropathy and Henoch–Schönlein purpura nephritis. Pediatr Res 89, 667–672 (2021). https://doi.org/10.1038/s41390-020-0951-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0951-1
This article is cited by
-
Glomerular expression and urinary excretion of fatty acid-binding protein 4 in IgA nephropathy
Journal of Nephrology (2023)
-
IgA glycosylation and immune complex formation in IgAN
Seminars in Immunopathology (2021)
-
Decreased glycolysis induced dysfunction of NK cells in Henoch-Schonlein purpura patients
BMC Immunology (2020)