Abstract
Background
To determine total sodium load, including inadvertent load, during the first 2 postnatal weeks, and its influence on serum sodium, morbidity, and mortality in extremely low birth weight (ELBW, birth weight <1000 g) infants and to calculate sodium replacement models.
Methods
Retrospective data analysis on ELBW infants with a gestational age <28 + 0/7 weeks.
Results
Ninety patients with a median birth weight of 718 g and a median gestational age of 24 + 6/7 weeks were included. Median sodium intake during the first 2 postnatal weeks was 10.2 mmol/kg/day, which was significantly higher than recommended (2–5 mmol/kg/day). Sodium intake did not affect the risk for hypernatremia. Each mmol of sodium intake during the first postnatal week was associated with an increased risk of bronchopulmonary dysplasia (45%) and higher-grade intraventricular hemorrhage (31%), during the second postnatal week for necrotizing enterocolitis (19%), and during both postnatal weeks of mortality (13%). Calculations of two sodium replacement models resulted in a decrease in sodium intake during the first postnatal week of 3.2 and 4.0 mmol/kg/day, respectively.
Conclusions
Sodium load during the first 2 postnatal weeks of ELBW infants was significantly higher than recommended owing to inadvertent sodium intake and was associated with a higher risk of subsequent morbidity and mortality, although the study design does not allow conclusions on causality. Replacement of 0.9% saline with alternative carrier solutions might reduce sodium intake.
Impact
-
Sodium intake in ELBW infants during the first 2 postnatal weeks was twofold to threefold higher than recommended; this was mainly caused by inadvertent sodium components.
-
High sodium intake is not related to severe hypernatremia but might be associated with a higher morbidity in terms of BPD, IVH, and NEC.
-
Inadvertent sodium load can be reduced by replacing high sodium-containing carrier solutions with high levels of sodium with alternative hypotonic and/or balanced fluids, model based.
Similar content being viewed by others
Introduction
Commencing at birth, the neonate’s water and electrolyte homeostasis encounters a physiological adaption to the extrauterine environment. In extremely low birth weight (ELBW, birth weight <1000 g) infants, this adaption is affected by immaturity of the kidney and epidermis, as well as by diseases associated with prematurity.1,2,3 Early postnatal adaption is marked by contraction of the extracellular volume (ECV) caused by transdermal water loss via the immature epidermis and by continuing natriuresis.4 The mechanisms responsible for increased urinary sodium losses in preterm infants are multifactorial. The immature kidney exhibits glomerulo-tubular imbalance, a physiologic state that is present when the glomerular filtration rate exceeds the reabsorptive capacity of the renal tubules. This imbalance can be attributed to numerous factors, including a preponderance of glomeruli compared with tubular structures, renal tubular immaturity, large ECV, and reduced oxygen availability. Avoidance of sodium supplementation is particularly important in very premature infants, who have increased water losses and for whom early administration of sodium supplementation is associated with an increased risk of hypernatremia.5,6 Retention of sodium promptly occurs due to limited sodium excretion capacity.4,7 During this period, ELBW infants are prone to developing hypernatremia, which is related to excessive transdermal water loss and/or excessive sodium intake, leading to sodium retention as described above.3,4,7,8,9 ELBW infants often require arterial lines, fluid replacement, or intravenous antibiotics. Primarily, near isotonic solutions containing sodium are used for fluid replacement or as carrier solution for parenteral medications. These solutions are potential sources of inadvertent sodium load, which may result in hypernatremia. Electrolyte imbalances or fluid overload during this postnatal adaption are associated with a higher risk of morbidities (bronchopulmonary dysplasia [BPD], necrotizing enterocolitis [NEC], patent ductus arteriosus [PDA], intraventricular hemorrhage [IVH]) and mortality.7,9,10,11,12,13 Moreover, an increased sodium intake (>3–4 mmol/kg/day) during the first days of life is associated with an increased risk for BPD and IVH.7,14,15,16 Therefore, the aim of this study was to determine and quantify not only the prescribed but also the inadvertent sodium intake during the first 2 weeks of life in ELBW infants. We investigated the association of sodium load with serum sodium, morbidity, and mortality. Suggestions for sodium replacement models were calculated to improve sodium management and to avoid inadvertent sodium intake in ELBW infants.
Methods
In a retrospective study from January 1 to December 31, 2016, we investigated the sodium intake of ELBW infants during the first 2 postnatal weeks. The study was conducted at the Department of Pediatrics, Division of Neonatology of the Medical University of Vienna and was approved by ethics committee (1540/2017).
Inclusion criteria: inborn, gestational age <28 + 0/7 weeks, birth weight <1000 g.
Exclusion criteria: death within 24 h of birth, acute kidney injury (serum creatinine >1.5 mg/dl), syndrome of inappropriate ADH secretion, adrenogenital syndrome, diabetes insipidus.
Outcome measures
Daily sodium and glucose intake during the first 2 weeks of life was calculated from the electronic patient record forms (ICIP Philips, Austria; SAP3® SAP, Austria; catoPAN™ Becton Dickson, Austria) and included the following: parenterally administered fluids, arterial lines, parenteral nutrition, carrier solutions, enemas, and enteral nutrition. Specific therapeutics is given in Table 1. According to our local modified less invasive surfactant administration (LISA) protocol,17 infants received arterial lines during the first 24 h of life, whenever feasible, as a standard measure to detect hypotension as soon as possible. Arterial lines were removed once the infant was hemodynamically stable for at least 24 h. Fluid intake was calculated according to the recommendations by the ESPGHAN and the American Academy of Pediatrics and adapted to daily weight, daily weight loss, and daily bedside echocardiography.3 To minimize transdermal fluid loss, double-wall incubators with a humidity of 85–90% were used exclusively, and the humidity was reduced by 5% per week. In addition, infants were placed on silver films and covered with plastic wraps during the first 10–14 days of life according to the cornification status of the premature skin. Fluid replacement therapy with 10–15 ml/kg of balanced fluids (Elo-mel isoton® containing 5.6 mg sodium/ml) gives a sodium load of 1.4–2.8 mmol/kg, whereas an arterial flush regimen with isotonic saline (containing 9 mg sodium/ml) at a rate of 0.5 ml/h results in 12 ml/day of fluid and an extra sodium load of 1.9 mmol/day. Serum sodium was measured daily during the first postnatal week in all infants and during the second postnatal week in most infants of the studied cohort via blood gas analysis. The highest measurement was used for analysis. Hypernatremia was defined as serum sodium >145 mmol/l8 and categorized into the categories of mild (146–150 mmol/l) and severe hypernatremia (>150 mmol/l). Data on maternal characteristics, demographics, morbidity, and mortality were obtained by reviewing the electronic charts. IVH was defined according to Papile,18 NEC according to Bell,19 preeclampsia/eclampsia according to Tranquilli et al.20 BPD was defined as an FiO2 of >0.21 at a gestational age of 36 + 0/7 weeks.21 A hemodynamically significant PDA was defined as treatment with Ibuprofen or surgical closure. A post hoc analysis of sodium and glucose intake in two theoretical sodium replacement models, where fluid therapy and carrier solutions were replaced with alternatives containing low or no sodium, was conducted for days 1–7 (Table 2).
Statistical analysis
The study’s primary outcome parameter was median sodium intake during the first 2 weeks of life (mmol/kg/day). Secondary outcome parameters were median sodium intake during the first and second postnatal week as well as for each day separately; main sources of sodium intake for each day; median glucose intake (mg/kg/min) and serum sodium levels (mmol/l) for the above stated periods; and the incidence of hypernatremia, BPD, IVH, NEC, PDA, and mortality. Statistical analysis was performed using IBM SPSS V.24.0.0.0 2016 (SPSS, Inc., Chicago, IL, USA) and R Core Team (2018; R: https://www.R-project.org/). Categorical data were summarized using absolute and relative frequencies. Continuous data were summarized using medians, means, and interquartile ranges. Wilcoxon signed-rank test was used to compare the median sodium and glucose intake of our cohort with a known standard value (the median of their recommended daily maximum intake, extracted from the ESPGHAN guidelines) for the given intervals (days 1–14, days 1–7, day 8–14). Correlation of sodium intake and maximum serum sodium of the next day were computed using a linear mixed-effect model. Associations between sodium intake for given intervals and morbidities and mortality were calculated using logistic regression and Cox regression, respectively. Data are presented as unadjusted odds ratios (ORs) and hazard ratios (HRs) with accompanying 95% confidence intervals (CIs). All tests were two sided, and a p value <0.05 was considered statistically significant.
Results
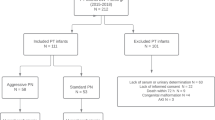
During the 1-year study period, 94 ELBW infants with a gestational age of <28 + 0/7 weeks were born at the Medical University of Vienna. Three infants died within the first 24 h of life and were excluded. One infant was excluded owing to acute renal failure during the first 2 weeks of life. In total, 90 infants were included in our final analysis.
Baseline characteristics, morbidity, and mortality
In our study population, median gestational age was 24 + 6/7 weeks (interquartile range (IQR) 24 + 0/7–26 + 2/7 weeks) and median birth weight 718 g (IQR 649–840 g). Data on weight change in relation to birth weight and change of weight z-scores are given in Supplementary Fig. S1. A median nadir of weight change was found at the fifth day of life, with a median weight loss of −4.2% (IQR −8.3 to 0.6%). Data on daily fluid intake are given in Supplementary Fig. S4. A summary of baseline characteristics together with the infants’ morbidities and mortality is given in Table 3. In addition, 36.7% (33/90) of study population were multiples, which is a higher percentage than reported in the literature (23%).22
Sodium intake
Sodium intake of ELBW infants during the first 2 weeks of life was 10.2 mmol/kg/day (median, IQR 9.0–12.6 mmol/kg/day) and therefore significantly higher (p < 0.01) than the recommended maximum daily intake of 5 mmol/kg/day.2,3 Separate analysis of the first and second postnatal week showed sodium intakes of 11.3 mmol/kg/day (median, IQR 9.3–14.1 mmol/kg/day) and 9.6 mmol/kg/day (median, IQR 7.5–12.6 mmol/kg/day), respectively. Daily sodium intake during the first 2 weeks of life is given in Fig. 1.
Table 2 and Fig. 2 show detailed sodium load from inadvertent sodium sources, such as ampicillin, gentamicin, arterial blood pressure measurement (ABPM), balanced fluid replacement therapy, and phosphate.
Hypernatremia
During the first postnatal week, 92.2% (83/90) of the infants developed hypernatremia and 64.4% (58/90) developed severe hypernatremia; incidence was lower in the second postnatal week (47.0% [39/83] and 16.7% [15/83], respectively). Maximum serum sodium levels occurred between the second and sixth day of life (Fig. 1). In a linear mixed-effect model, a 1 mmol/kg increase of sodium intake was associated with an average serum sodium increase of 0.05 mmol/l (95% CI −0.03–0.15, n.s.) on the following day during the first 2 weeks of life (Fig. 3).
Glucose intake
Glucose intake of ELBW infants during the first 2 weeks of life (median 7.55 mg/kg/min, IQR 6.20–8.59 mg/kg/min) always stayed in the recommended daily intake range (5.5–10.4 mg/kg/min).2,3 Separate analysis of the first and second postnatal week showed a glucose intake of 6.52 mg/kg/min (median, IQR 5.45–7.90 mg/kg/min) during week 1 and a glucose intake of 8.34 mg/kg/min (median, IQR 6.82–10.28 mg/kg/min) during week 2. Median glucose intake deriving from parenteral nutrition during the first and second postnatal week was 4.48 mg/kg/min (range 1.07–8.06 mg/kg/min) and 4.37 mg/kg/min (range 0.1–8.71 mg/kg/min), respectively.
Association of sodium intake and morbidity and mortality
The intake of each mmol of sodium in ELBW infants during the first postnatal week was associated with a 45% higher risk of BPD (OR 1.45, p < 0.01) and a 31% higher risk of IVH grade III or IV (OR 1.31, p < 0.01). However, sodium intake during the first postnatal week was not associated with an increased risk for hypernatremia (OR 1.03 and 0.99, n.s.). During the second postnatal week, intake of each mmol of sodium was associated with an increase in the ELBW infant’s risk of BPD by 19% (OR 1.19, p < 0.05) and of NEC by 19% (OR 1.19, p < 0.01). Similarly, to week 1, sodium intake during the second postnatal week was not associated with an increased risk for hypernatremia (OR 0.97 and 1.08, n.s.). During the first 2 postnatal weeks, each mmol of sodium intake was associated with a 13% higher risk for death (HR 1.13, p < 0.01). The impact of sodium intake on morbidity and mortality is summarized in Fig. 4. Supplementary Fig. S2 shows parameters of the red blood cell line, blood transfusions, sodium intake, serum sodium, and fluid therapy 12–48 h before IVH was diagnosed. There was no relevant hematocrit drop, sodium load, or higher need for fluid therapy 12–48 h before IVH was detected for the first time. Supplementary Fig. S3 shows sodium intake, serum sodium, and fluid therapy 2–48 h before NEC and at NEC onset. There was no relevant sodium load or higher need for fluid therapy 2–48 h before NEC onset was diagnosed.
Sodium replacement models
In a post hoc analysis, we calculated two theoretical sodium replacement models for the first postnatal week, where carrier solutions of specific medications and fluid therapy were changed; this information is given in Table 2. In model 1, median sodium intake was decreased by 3.2 mmol/kg/day (from 11.3 to 8.1 mmol/kg/day). In model 2, median sodium intake was decreased by 4.0 mmol/kg/day (from 11.3 to 7.3 mmol/kg/day). Both models increase the median glucose intake. In model 1, this increase is 0.73 mg/kg/min from 6.52 to 7.25 mg/kg/min, and in model 2 this increase is 1.2 mg/kg/min from 6.52 to 7.72 mg/kg/min. Glucose intake, however, remains within the recommended range (5.5–10.4 mg/kg/min).2,3
Discussion
In a retrospective explorative analysis, we investigated the effective sodium intake in ELBW infants during their first 2 postnatal weeks and its impact on hypernatremia, morbidity, and mortality. Effective median sodium intake was 10.2 mmol/kg/day and therefore significantly (p < 0.01) higher than the currently published recommendations (2–5 mmol/kg/day).2,3 Sodium intake did not affect the risk for hypernatremia, but it was associated with a significantly increased risk of BPD, higher-grade IVH, NEC, and mortality (p < 0.01). However, the study design does not allow conclusions on causality. To avoid excessive sodium intake in future, we calculated two theoretical sodium replacement models with alternative carrier solutions, both of which turned out to be feasible and appropriate in theoretical calculations.
Sources of sodium intake
In the present study, parenteral nutrition and sodium was administered according to ESPGHANS guidelines for preterm infants. However, >92% of our patients had a daily sodium intake above the recommended intake. This was related to inadvertent sodium intake caused by carrier solutions from antibiotics, fluid replacement therapy, and ABPM: almost all ELBW infants received antibiotic prophylaxis with ampicillin and gentamicin for the first 48 h of life. Furthermore, infants had arterial lines placed in accordance with the LISA protocol.17 These are continuously flushed with heparin and 0.9% saline (Table 2). If patients were treated under this standard treatment plan, consisting of antibiotics, ABPM, and parenteral nutrition, a 500-g infant received 9.5 mmol/kg/day of sodium during the first postnatal week, which is almost three times the recommended intake of 0–3 mmol/kg/day. If an infant received fluid replacement therapy with balanced crystalloids to treat conditions such as mild hypotension, a median of 10–15 ml/kg of crystalloid extra given resulted in an extra median sodium intake of 2.8 mmol/kg/day. During the second week, the sources of extra sodium differed slightly, as antibiotics and ABPM were completed, whereas phosphate, enteral nutrition, and fluid replacement therapy for management of hypotension were continued and thus became the dominating sodium-containing agents. Blood transfusions were recently described as a rather important source of inadvertent sodium loads.23 In our analysis, blood transfusions accounted for a mean of <6% of the total sodium intake each day during the first 2 weeks of life. This extra sodium intake caused by inadvertent sodium sources is usually not reviewed by the electronic patient documentation system, and it therefore is likely that clinicians accidentally exceed the recommended daily intake in this group of very small and premature patients.
Hypernatremia
Owing to the ELBW infants’ limited renal sodium excretion capacity in response to high sodium loads, excessive sodium intake during the first weeks of life can lead to sodium retention.2,7,8 We found a non-significant (n.s.) and clinically irrelevant increase of 0.05 mmol/l in serum sodium for each mmol/kg sodium received the day before. Nevertheless, sodium intake did not affect the incidence of hypernatremia. We hypothesize that this effect is related to a combination of mutually reinforcing processes. Primarily, excessive sodium intake leads to renal retention of sodium with subsequent water retention in the ECV.7,13,14,15,24 Measures taken to reduce the excessive insensible water loss in the ELBW infant such as high incubator and ventilator humidity contribute to further fluid overload resulting in serum sodium dilution. Second, we hypothesize that most of the administered sodium is stored in interstitial tissue and endothelial cells. The interstitial tissue contains negatively charged glycosaminoglycans that are capable of binding and storing osmotically inactive sodium (Fig. 5). The intravascular endothelial glycocalyx is also able to bind sodium and store it in endothelial cells (Fig. 6). An excessively high sodium concentration in the interstitial glycosaminoglycans and endothelial glycocalyx results in the loss of its buffering capacity and disruption of their structure with further osmotically active sodium transport and concomitant fluid shifts into the interstitial tissue.25,26,27 This results in interstitial edema possibly aggravated by the inhibition of lymphatic drainage of the interstitial fluids caused by an elevation of natriuretic peptides through high sodium intake.28
Model of interstitial sodium accumulation and disruption of its glycosaminoglycan network through high sodium intake (adapted from Nijst et al. with permission of the authors).27
Model of endothelial dysfunction and breakdown of the glycocalyx through high sodium intake (adapted from Nijst et al. with permission of the authors).27
Morbidity and mortality
We hypothesize that sodium and fluid shifts into the interstitial tissue of the lung, as described above, may lead to pulmonary edema, which could impair oxygenation in ELBW infants. Oxygenation problems lead to respiratory failure requiring mechanical ventilation, which is a key player in the pathogenesis of BPD.13 In the present study, each mmol of sodium intake during the first postnatal week was associated with an increased risk of BPD by 45% (p < 0.01). This is in line with other clinical studies that found a significant association between higher sodium intake and increased risk of BPD in infants with a gestational age of ≤30 + 0/7 weeks.9,14,15 Furthermore, fluid overload and insufficient contraction of the ECV, as part of the postnatal adaption, are associated with higher risk for BPD.7,10,12,13 Fluid and sodium shifts, as described above, may also affect cerebral and intestinal blood flow.29,30,31,32 We hypothesize that, sodium overload of the intracerebral endothelial cells could lead to autonomous vasoconstriction, through interference with nitric oxide (NO) production and endothelial stiffness, followed by fluid shifts from the venous reservoir into effective circulation (Fig. 6).27 These cerebral fluid fluctuations particularly within the fragile vessels of the periventricular matrix might lead to development of IVH.30 Subsequently, we found an association for risk increment of higher-grade IVH of 31% (p < 0.01) for each mmol of sodium intake during the first postnatal week. Furthermore, we hypothesize that the same autonomous vasoconstrictive and NO-interfering mechanisms might lead to disturbances of autoregulation in the mesenteric vessels leading to intestinal ischemia and ultimately to NEC.33,34 Accordingly, each mmol of sodium intake during the second postnatal week was associated with an increased risk for NEC by 19% (p < 0.01). High sodium intake, associated fluid shifts, and disturbances in vascular autoregulation were associated with an increased risk for both morbidity and mortality, by 13% (p < 0.01) during the first 2 postnatal weeks. On the basis of our findings, we propose a hypothetic pathophysiological model to encompass all found associations for morbidity and mortality in one explanatory, previously described in adults with cardiac morbidity, pathophysiological process.
Sodium replacement models
To avoid excessive sodium intake in EBLW infants, we calculated two sodium replacement models. Therefore, the top four sodium sources consisting of carrier solutions and fluid therapy were replaced by alternatives containing either less or no sodium (Table 2). Unfortunately, alternatives for sodium glycero-phosphate are unavailable in most parts of Europe. Model 1 achieved a reduction of median sodium intake by 3.2 mmol/kg/day. An even better reduction of 4.0 mmol/kg/day can be achieved by using model 2. Both models increased median glucose intake by 0.73 mg/kg/min (model 1) and 1.2 mg/kg/min (model 2), respectively, but the cumulative glucose intake consisting of parenteral nutrition and the replacement models stayed within the ESPGHAN’s recommendations for daily glucose intake. If glucose is infused via an arterial line, the amount of glucose in the parenteral nutrition solution can potentially be reduced and more volume remains for other nutrients, if non-standard bags are used. Other options for alternative carrier solutions of arterial lines are isotonic protein solutions.35,36 A replacement of 0.9% saline by isotonic amino acid solutions might improve amino acid intake and accelerate protein synthesis.37 Furthermore, less hemolysis was observed in experiments using isotonic amino acid solutions in comparison to 0.9% saline.36 In our models, fluid therapy was replaced by hypotonic crystalloids (0.07 mmol/ml sodium, 50 mg/ml glucose). Recent literature recommends the use of 0.3%/0.45% saline + 5% glucose for fluid maintenance therapy, especially for hypernatremic term infants, because sodium intake would be equivalent to the newborn’s renal sodium excretion (0.5 mmol/kg/h).38 Semitone fluids add glucose to the ELBW infants’ low glycogen storages and free water, which is initially lost by insensible water loss.1,39
Strengths and limitations
This study confirms previous findings of other studies on determination of inadvertent sodium intake, with a very detailed breakdown of determination and quantification of effective and inadvertent sodium intake in the population of ELBW infants.23,40 Given the observational and retrospective character of the study, we can only establish associations between sodium load and morbidity and mortality in ELBW infants and not causal correlations.
Conclusion
Sodium intake in ELBW infants during the first 14 postnatal days was significantly higher than recommended owing to inadvertent sodium sources. Furthermore, we observed an association of higher sodium intake with an increased risk of morbidity (BPD, higher-grade IVH, NEC) and mortality. However, as the final causality remains unclear these findings should be confirmed in future randomized controlled trials. Replacement of 0.9% saline with alternative carrier solutions (e.g., 5% glucose) might reduce sodium intake. To improve clinicians’ awareness of hidden sodium sources, sodium intake through phosphate and ABPM should be visualized in electronic documentation records and also implemented into nutrient calculations. This might ensure a more physiologic adaption of the ELBW infants’ water and electrolyte homeostasis.
References
Baumgart, S. & Costarino, A. T. Water and electrolyte metabolism of the micropremie. Clin. Perinatol. 27, 131–146 (2000).
Koletzko, B., Poindexter, B. & Uauy, R. Nutritional Care of Preterm Infants: Scientific Basis and Practical Guidelines (S. Karger AG, 2014).
Tsang, R. C. Nutrition of the Preterm Infant: Scientific Basis and Practical Guidelines (Digital Educational Publishing, Incorporated, 2005).
Modi, N. Sodium intake and preterm babies. Arch. Dis. Child. 69, 87–91 (1993).
Martin, R. J., Fanaroff, A. A. & Walsh, M. C. Fanaroff and Martin’s Neonatal-Perinatal Medicine E-Book: Diseases of the Fetus and Infant (Elsevier Health Sciences, 2014).
Robillard, J. E., Segar, J. L., Smith, F. G. & Jose, P. A. Regulation of sodium metabolism and extracellular fluid volume during development. Clin. Perinatol. 19, 15–31 (1992).
Costarino, A. T. Jr., Gruskay, J. A., Corcoran, L., Polin, R. A. & Baumgart, S. Sodium restriction versus daily maintenance replacement in very low birth weight premature neonates: a randomized, blind therapeutic trial. J. Pediatr. 120, 99–106 (1992).
Bockenhauer, D. & Zieg, J. Electrolyte disorders. Clin. Perinatol. 41, 575–590 (2014).
Gawlowski, Z., Aladangady, N. & Coen, P. G. Hypernatraemia in preterm infants born at less than 27 weeks gestation. J. Paediatr. Child Health 42, 771–774 (2006).
Bell, E. F. & Acarregui, M. J. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. CD000503 (2014).
Dalton, J., Dechert, R. E. & Sarkar, S. Assessment of association between rapid fluctuations in serum sodium and intraventricular hemorrhage in hypernatremic preterm infants. Am. J. Perinatol. 32, 795–802 (2015).
Kair, L. R., Leonard, D. T. & Anderson, J. M. Bronchopulmonary dysplasia. Pediatr. Rev. 33, 255–263 (2012). quiz 263–264.
Oh, W. et al. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J. Pediatr. 147, 786–790 (2005).
Hartnoll, G., Betremieux, P. & Modi, N. Randomised controlled trial of postnatal sodium supplementation on oxygen dependency and body weight in 25-30 week gestational age infants. Arch. Dis. Child. Fetal Neonatal Ed. 82, F19–F23 (2000).
Hartnoll, G., Betremieux, P. & Modi, N. Randomised controlled trial of postnatal sodium supplementation on body composition in 25 to 30 week gestational age infants. Arch. Dis. Child. Fetal Neonatal Ed. 82, F24–F28 (2000).
Lee, H. J. et al. Early sodium and fluid intake and severe intraventricular hemorrhage in extremely low birth weight infants. J. Korean Med. Sci. 30, 283–289 (2015).
Klebermass-Schrehof, K. et al. Less invasive surfactant administration in extremely preterm infants: impact on mortality and morbidity. Neonatology 103, 252–258 (2013).
Volpe, J. Neurology of the Newborn 4th edn (Saunders, Philadelphia, 2001).
Kliegman, R. M. & Walsh, M. C. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr. Probl. Pediatr. 17, 213–288 (1987).
Tranquilli, A. L. et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 4, 97–104 (2014).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729 (2001).
Vohr, B. R. et al. Maternal age, multiple birth, and extremely low birth weight infants. J. Pediatr. 154, 498–503 (2009).
Spath, C., Sjostrom, E. S., Ahlsson, F., Agren, J. & Domellof, M. Sodium supply influences plasma sodium concentration and the risks of hyper- and hyponatremia in extremely preterm infants. Pediatr. Res. 81, 455–460 (2017).
O’Brien, F. & Walker, I. A. Fluid homeostasis in the neonate. Paediatr. Anaesth. 24, 49–59 (2014).
Wiig, H., Luft, F. C. & Titze, J. M. The interstitium conducts extrarenal storage of sodium and represents a third compartment essential for extracellular volume and blood pressure homeostasis. Acta Physiol. (Oxf.) 222, e13006 (2018).
Olde Engberink, R. H. et al. Role of the vascular wall in sodium homeostasis and salt sensitivity. J. Am. Soc. Nephrol. 26, 777–783 (2015).
Nijst, P. et al. The pathophysiological role of interstitial sodium in heart failure. J. Am. Coll. Cardiol. 65, 378–388 (2015).
Marik, P. E. Iatrogenic salt water drowning and the hazards of a high central venous pressure. Ann. Intensive Care 4, 21 (2014).
Adrogue, H. J. & Madias, N. E. Hypernatremia. N. Engl. J. Med. 342, 1493–1499 (2000).
Ballabh, P. Pathogenesis and prevention of intraventricular hemorrhage. Clin. Perinatol. 41, 47–67 (2014).
Sterns, R. H. Disorders of plasma sodium–causes, consequences, and correction. N. Engl. J. Med. 372, 55–65 (2015).
Roze, J. C. et al. Nutritional strategies and gut microbiota composition as risk factors for necrotizing enterocolitis in very-preterm infants. Am. J. Clin. Nutr. 106, 821–830 (2017).
Polin, R. A., Abman, S. H., Rowitch, D. & Benitz, W. E. U. Fetal and Neonatal Physiology (Elsevier Health Sciences, 2016).
Watkins, D. J. & Besner, G. E. The role of the intestinal microcirculation in necrotizing enterocolitis. Semin. Pediatr. Surg. 22, 83–87 (2013).
Jackson, J. K. et al. Can an alternative umbilical arterial catheter solution and flush regimen decrease iatrogenic hemolysis while enhancing nutrition? A double-blind, randomized, clinical trial comparing an isotonic amino acid with a hypotonic salt infusion. Pediatrics 114, 377–383 (2004).
Jackson, J. K. & Derleth, D. P. Effects of various arterial infusion solutions on red blood cells in the newborn. Arch. Dis. Child. Fetal Neonatal Ed. 83, F130–F134 (2000).
Moltu, S. J. et al. Enhanced feeding and diminished postnatal growth failure in very-low-birth-weight infants. J. Pediatr. Gastroenterol. Nutr. 58, 344–351 (2014).
National Clinical Guideline Centre. IV Fluids in Children: Intravenous Fluid Therapy in Children and Young People in Hospital (National Institute for Health and Care Excellence (UK), London, 2015).
Hay, W. W. Early postnatal nutritional requirements of the very preterm infant based on a presentation at the NICHD-AAP workshop on research in neonatology. J. Perinatol. 26(Suppl 2), S13–S18 (2006).
Kermorvant-Duchemin, E. et al. Early chloride intake does not parallel that of sodium in extremely-low-birth-weight infants and may impair neonatal outcomes. J. Pediatr. Gastroenterol. Nutr. 54, 613–619 (2012).
Author information
Authors and Affiliations
Contributions
Investigation, F.E., G.L.-W. (pharmacology); writing, F.E., original draft preparation F.E.; visualization, F.E., R.R.; software, F.E.; conceptualization, N.H., F.E.; methodology, N.H., G.L.-W. (pharmacology); resources, N.H.; data curation, N.H., M.T.; statistics, R.R.; writing, review, and editing, B.J., S.E., G.L.-W. (pharmacology); supervision, A.B.; project administration, F.E.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Owing to the study’s retrospective character, no informed consent from the included patients was required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Eibensteiner, F., Laml-Wallner, G., Thanhaeuser, M. et al. ELBW infants receive inadvertent sodium load above the recommended intake. Pediatr Res 88, 412–420 (2020). https://doi.org/10.1038/s41390-020-0867-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0867-9
This article is cited by
-
Neurodevelopmental consequences of early plasma sodium changes in very preterm infants
Pediatric Research (2022)
-
Optimising insulin aspart practices in a neonatal intensive care unit: a clinical and pharmaco-technical study
European Journal of Pediatrics (2021)
-
Is inadvertent electrolyte overload in very preterm infants preventable?
Pediatric Research (2020)