Abstract
Background
Oropharyngeal colostrum (OC) is a novel feeding strategy to prevent complications of prematurity. A meta-analysis was conducted to investigate whether very low birth weight infants (VLBWs) can benefit from OC.
Methods
Randomized controlled trials (RCTs) were searched from Embase, PubMed, Web of Science, and Cochrane Central Register of Controlled Trials from the date of inception until May 2019. RCTs were eligible if they used OC therapy on VLBW infants. The primary outcomes included ventilator-associated pneumonia (VAP), necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), late-onset sepsis, and death. The secondary outcomes included the time of full enteral feeding and the length of stay.
Results
Eight RCTs involving 682 patients (OC group: 332; non-OC group: 350) were included in the meta-analysis. The results suggested that OC was associated with a significantly reduced incidence of VAP [odds ratio (OR) = 0.39, 95% confidence interval (CI): 0.17–0.88, P = 0.02] and full enteral feeding days (mean difference = −2.66, 95% CI: −4.51 to −0.80, P = 0.005), a potential significance of NEC (OR = 0.51, 95% CI: 0.26–0.99, P = 0.05), a trend toward downregulating mortality (OR = 0.60, 95% CI: 0.34–1.08, P = 0.09) and proven sepsis (OR = 0.64, 95% CI: 0.40–1.01, P = 0.06).
Conclusions
OC could significantly reduce the occurrence of VAP, and consequently, its routine use should be considered for VLBWs to prevent infectious diseases.
Impact
-
OC significantly reduces the occurrence of VAP and NEC in VLBW infants.
-
OC may reduce the incidence of VAP and NEC by increasing IgA levels.
-
Early OC therapy for mechanical ventilation of low-weight infants may prevent the occurrence of VAP.
Similar content being viewed by others
Introduction
Premature infants, especially very low birth weight (VLBW) infants (<1500 g), are a group at high risk of infectious diseases in neonatal intensive care units (NICUs), and such infections impose a great socioeconomic burden and account for 23% of total newborn health care costs.1,2,3 Ventilator-associated pneumonia (VAP), a frequent and severe complication in premature infants on mechanical ventilation, is considered the second most common infectious disease in the NICU, ranked behind central line-associated bloodstream infections.4,5 Considering the longer hospital stay and rising medical cost, prophylaxis of VAP is required.
Low immune function in VLBWs is a high-risk factor for VAP.6,7 The smaller the gestational age, the lower the birth weight and the higher incidence of VAP will be, suggesting that immune function and lung maturity have a closer relationship with VAP.7,8 Recent studies found that immunoglobulins are vital to reducing the risk of ventilator-related infections,9 and there are considerable immunoglobulins in human milk.
Human milk is recognized as the most beneficial form of nutrition for infants, and the concentration of immune factors is much higher in colostrum than in mature milk.10 Colostrum, secreted 2 to 3 days after birth, is rich in immunoglobulins (e.g., IgG, IgA, IgM and lactoferrin), of which secretory IgA (SIgA) is the highest in all exocrine fluids.10 It has been shown that colostrum appears to lower the risk of late-onset sepsis, feeding intolerance, and other complications of preterm labor.10,11
In critically ill adult patients, oropharyngeal administration of probiotics is capable of effectively alleviating oral contamination and reducing the incidence of VAP, suggesting that the oropharyngeal mucosa can effectively stimulate immune activation.12,13,14 Recent studies on oral and pharyngeal colostrum administration in preterm infants have revealed a rise in the number of immune components in preterm infants. Based on this evidence, NICUs have begun colostrum therapy for premature infants to further enhance immune protection.15,16
Oropharyngeal colostrum (OC) is a novel feeding strategy to prevent complications of prematurity. Previous studies revealed that the OC procedure is easy to implement and does not require enteral feeding.16 The breast milk required is small in amount for the baby to swallow. A small amount of milk (usually 0.2 mL divided into two cheeks) is placed on the mucous membrane of the mouth for absorption. Sterile cotton swabs or oral applicators are used for each application of human milk.17 Colostrum contacts the mouth and oropharyngeal pouch and produces immune protection and stimulation of oropharyngeal receptors by the abundant anti-inflammatory and pro-inflammatory cytokines detected in the mother’s colostrum and milk. Gastrointestinal movement, secretion, and absorption capacity are enhanced.18
Previous studies have suggested that OC can effectively increase the immune factors of premature infants and has major implications for preventing complications of prematurity.19,20,21,22,23,24,25,26,27,28,29
From the perspective of the author, this meta-analysis focused on investigating colostrum’s connection with the clinical complications of VLBW infants (<32 weeks gestation and/or <1500 g birth weight) as well as the clinical and economic benefits. Accordingly, a meta-analysis and a systematic review were conducted to investigate whether VLBW infants can benefit from the administration of OC.
Methods
This meta-analysis of the existing literature was conducted in line with the Preferred Reporting Items for the guidelines of Meta-Analyses and Systematic Reviews.30 The PRISMA checklist is shown in an additional file (see Additional file 1).
Literature search strategy
The electronic literature databases were searched as follows: Embase, PubMed, Web of Science, and Cochrane Central Register of Controlled Trials from their date of inception to May 2019. The following search terms were used as keywords to identify all relevant studies: “oropharyngeal colostrum” or “colostrum” or “human milk” and “newborn” or “VLBW infant” or “low birth weight infant” or “premature infant” or “neonate.” Related papers that were listed in the references and included these keywords were also examined for potentially qualified studies.
Inclusion and exclusion criteria
Studies with the following criteria were included:
-
1.
Type of trials: Randomized controlled trials (RCTs).
-
2.
Population: Preterm infants delivered at <32 weeks gestation and/or <1500 g birth weight.
-
3.
Intervention: Administration of OC to premature infants.
-
4.
Comparison: Placebo alone (saline) or no intervention.
-
5.
Outcome: Primary outcomes (e.g., VAP, necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), proven sepsis, clinical sepsis, late-onset sepsis, and death) and secondary outcomes (e.g., length of stay and time of full enteral feeding).
Studies with the following criteria were excluded:
-
1.
Not published in English.
-
2.
Not published as original articles.
-
3.
Uncontrolled, pseudorandomized, retrospective studies, reviews, systematic reviews, meta-analyses, case reports, letters, correspondences, comments, or editorials.
-
4.
Including no data on clinical outcomes in the premature infant.
-
5.
Full text of the article not available.
Data extraction and quality assessment
Two reviewers assessed the data from the included studies. Through discussion, the differences between the two reviewers were resolved. The extracted data included the study design, the source of patients, publication year, the author, and detailed clinical outcomes (VAP, NEC, BPD, ROP, death, length of stay, time of full enteral feeding, proven sepsis, clinical sepsis, late-onset sepsis) stratified by OC. Evaluation of research quality was based on six basic criteria of the Cochrane Bias Risk Assessment Tool: selective outcome reporting, incomplete outcome data, blinding of outcome assessors, allocation concealment, sequence generation, and other bias sources.31
Statistical analysis
Every statistical analysis was conducted by adopting the odds ratio (OR), and Review Manager (RevMan, version 5.3.5) with 95% confidence intervals (CIs) was used to assess the effect of OC and the incidence of diseases. Odds ratios (ORs) or mean differences (MDs) with 95% CIs were employed to assess the association between the clinical outcomes and OC administration for categorical or continuous variables, respectively. Heterogeneity in the study was assessed by the I2 tests and Cochran’s Q statistic.32 The random-effects model was adopted when heterogeneity was significant (I2 > 50%). Otherwise, the model with settled effects was adopted. Sensitivity analysis was guided by dropping out every study in a sequential way. The strategies of Begg and Egger were used to assess publication bias,33 through which an assessment of funnel-plot asymmetry was achieved.
Results
Study characteristics
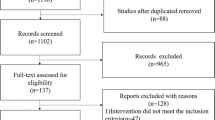
In our literature search, 204 potentially relevant citations were identified. Of these, 12 references that might be included in the meta-analysis were retrieved for further consideration.15,19,20,21,22,23,24,25,26,27,28,29 However, three of these 12 items were retrospective studies,15,19,20 and one21 was a non‐randomized interventional study. Therefore, eight RCTs finally met our criteria and were suitable for inclusion in the meta-analysis.22,23,24,25,26,27,28,29 The article selection is summarized in Fig. 1. Three studies were conducted in the United States, and the other five studies were conducted in Egypt, Brazil, China, India, and Korea. All the studies were RCTs. The sample size was 682 in total (OC group: 332; non-OC group: 350). Three studies23,24,27 achieved a double-blind controlled design, and other trials were single blinded. The included studies’ features are listed in Table 1, and each included study’s particular quality scores are shown in Fig. 2. The characteristics of feeding are listed in Table 2.
Ventilator-associated pneumonia
Of the eight studies, three reported VAP in the included infants. The aggregated results of these three studies suggested that low birth weight infants with OC administration presented a noticeable reduction in the incidence of VAP (OR = 0.39, 95% CI: 0.17–0.88, P = 0.02) (Fig. 3), and the test result for heterogeneity was mild (P = 0.26, I2 = 26%).
Necrotizing enterocolitis and bronchopulmonary dysplasia
All eight studies obtained data regarding NEC, in which the differences were of potential statistical significance (OR = 0.51, 95% CI: 0.26–0.99, P = 0.05), and the test result for heterogeneity was ascertained to be non-significant (P = 0.60, I2 = 0%). Four studies19,21,23,25 suggested data on BPD, and the different points in BPD were not statistically significant (OR = 0.77, 95% CI: 0.48–1.24, P = 0.28).
Death
Five studies22,23,25,27,29 obtained data on death, and the analysis results suggested great statistical trends (OR = 0.60, 95% CI: 0.34–1.08, P = 0.09). The findings revealed that OC was associated with a decrease in mortality. However, the results were of no statistical significance.
Proven sepsis, clinical sepsis, and late-onset sepsis
Proven sepsis was defined as clinical sepsis plus biologic markers of systemic inflammatory response syndrome (altered leukocyte count, C-reactive protein >12 mg/L) and positive blood culture. The effect of OC on proven sepsis in premature infants was assessed according to seven trials,22,23,24,25,26,27,28 and the heterogeneity was ascertained to be non-significant (P = 0.97, I2 = 0%). Although the results were not statistically significant, we still observed a decreasing trend of proven sepsis (OR = 0.64, 95% CI: 0.40–1.01, P = 0.06).
Clinical sepsis was defined as clinical signs of infection with antibiotic treatment for over three days.34 Four studies22,24,27,28 presented data on clinical sepsis, exhibiting moderately significant statistical heterogeneity between studies (P = 0.04, I2 = 63%). However, the difference in clinical sepsis was not statistically significant (OR = 0.54, 95% CI: 0.19–1.54, P = 0.25). Three studies23,25,26 showed data on late-onset sepsis, and the different points were not statistically significant (OR = 0.71, 95% CI: 0.40–1.27, P = 0.25). All the results are listed in Table 3.
Length of stay
Overall, there were six studies covering 331 infants investigating the intervening effect of OC on length of stay.22,23,25,26,27,29 However, the results were not statistically significant (MD = −4.68, 95% CI: −12.52 to 3.15), P = 0.24).
Time of full enteral feeding
Seven studies22,23,24,25,27,28,29 investigated whether OC can reduce the time of full enteral feeding, and the evidence of significant heterogeneity was obvious (P < 0.001, I2 = 87%). The results showed that OC indeed significantly shortened the length of full enteral feeding time (MD = −2.66, 95% CI: −4.51 to −0.80, P = 0.005) (Fig. 4).
Discussion
Our meta-analysis suggested that OC was associated with a significant reduction in the incidence of VAP and shortened the full enteral feeding days, with a potential significance of reducing the incidence of NEC and a trend toward downregulating the ICU mortality and the incidence of proven sepsis. However, the results revealed no statistically significant change in the length of ICU stay, BPD, ROP, or late-onset sepsis. The clinical evidence regarding the strengths of OC administration in VLBW infants has great implications for neonatal infection-related diseases. We could further concentrate our interest on the economic and clinical benefits of OC.
Previous studies16,17 demonstrated the safety and feasibility of oral care with colostrum. The whole process was very safe, and there were no adverse events associated with this procedure. A sterile cotton swab was dipped in milk (for which the estimated amount of milk was 0.2 mL), and the cotton was placed in the infant’s mouth close to the oropharynx. The transfer of sIgA and lactoferrin was evident in the urine and tracheal aspirates.
Lee et al.27 and Rodriguez’s29 studies observed increased levels of circulating immune-protective factors (IgA, IgM, and lactoferrin) in urine and saliva and an inhibition of the secretion of pro-inflammatory cytokines of extremely premature infants submitted to colostrum oropharyngeal administration, directly proving that colostrum can increase neonatal immune factors and promote immunity.
It is generally known that the occurrence of VAP in premature infants is largely attributed to the immaturity of the immune system. In addition, a recent meta-analysis of the risk factors for VAP in neonates reported that low birth weight, prematurity, and parenteral nutrition were independent risk factors for VAP development.7
The major findings of our meta-analysis evidenced the protective factors of oral colostrum against the development of VAP. There are many risk factors affecting the occurrence of VAP, among which one of the most important factors is the duration of mechanical ventilation.7 We summarized the baseline risk for VAP in Table 1, and the baselines of mechanical ventilation in the intervention and control groups were consistent. As an outcome indicator, Abd-Elgawad’s study22 showed that colostrum could significantly shorten the length of mechanical ventilation, which indirectly proved our point. Although we would like to extract more data on mechanical ventilation for deeper discussion, the lack of raw data in the article is our main difficulty, and it appears that most articles have not shifted their focus to the benefits of oral colostrum in premature infants with mechanical ventilation. Thibeau’s study,15 which we excluded because it is a retrospective study, directly investigated human milk and mechanical ventilation-related pneumonia, and there were no statistically significant differences. Since this study is not an RCT, the evidence is not very strong. Thus, we expect that more RCTs will be conducted on the relationship between VAP and colostrum.
NEC is a devastating intestinal inflammatory disease that can cause necrosis and perforation of the intestinal epithelium, with significant morbidity and mortality.35,36 The prevention of NEC has become one of the most important topics of interest to researchers. In our meta-analysis, we found that intervention with OC can potentially reduce the occurrence of NEC (OR = 0.51, 95% CI: 0.26–0.99, P = 0.05). The p value is exactly equal to 0.05, and the confidence interval of the OR did not include 1, so we considered the result as potentially statistically significant. Although the pathophysiology of NEC is still not completely understood, recent research shows that maternal IgA protects against the development of NEC in preterm infants.37 Colostrum is rich in immunoglobulins (e.g., IgG, IgA, IgM, and lactoferrin), of which SIgA is the highest in all exocrine fluids.10 This also reasonably explains our results. Although the incidence of NEC was not statistically significant in the eight articles included, it was observed that the intervention group had a slight downward trend in each study, which may also be the reason for our potential statistical significance. Hence, we still need larger sample sizes of RCTs to continue to confirm the preventive effect of OC.
However, the incidence of sepsis, an immune-related high-risk neonatal disease (proven sepsis, clinical sepsis, and late-onset sepsis), was not statistically significant. We believe there are two possible reasons: first, Moreno’s study21 suggested that the sIgA of the colostrum group continued to increase over the 30 days of the observation period and was higher than that of the control group, while the lactoferrin content decreased after reaching its peak at 15 days. This reveals that the stimulating effect of colostrum on lactoferrin is not persistent. Previous studies revealed that low neonatal IgA levels were a risk factor for VAP,38 and lactoferrin levels were associated with sepsis.39 This can explain why in this meta-analysis, the occurrence of VAP was statistically significant, and sepsis only displayed a downward trend. Second, colostrum contact between the oropharyngeal mucosa and this fluid may have immunomodulatory effects resulting in a decrease in inflammation. The non-statistical significance of colostrum administration for sepsis may indicate that colostrum has an upregulating effect on the immune function of newborns, but may overexaggerate the downregulatory ability of inflammation. In this regard, colostrum administration may facilitate the prevention of VAP. Although we just found that OC was associated with a trend toward downregulating proven sepsis, we believe that low birth weight infants with proven sepsis may benefit from OC, so we hope that future studies will pay more attention to this part of the population and finally obtain a more certain answer.
It was found that OC administration significantly reduced the time to reach full enteral feeding, which agrees with five studies.22,24,27,29 Such a finding is of great implication, since it shows that early enteral feeding of breast milk has a long-term beneficial effect on intestinal atrophy, lowering the risk of local inflammation, feeding intolerance, NEC, and hospital infection.16 Accordingly, colostrum administration to preterm infants who cannot be fed intestinally may be a potential immunotherapy for VLBW infants.
In addition, we need to attach importance to the feeding type, breast milk consumption, and duration of breastmilk feeding. Five articles mentioned feeding strategies for oral colostrum administration and breast milk intake between the intervention and control groups (Table 2). Although there was no significant difference in breast milk intake, the breast milk consumption in the control group was slightly higher than that in the intervention group, indicating that OC can achieve better clinical outcomes with a smaller amount, which is a good result.
Until now, two meta-analyses, namely, Garg’s40 and Panchal’s41 studies, have investigated the intervention of OC therapy. In fact, both our study and previous meta-analysis have analyzed the clinical outcome of NEC and BPD and have demonstrated that the full time of enteral feeding is associated with OC. However, there are also some differences between our study and the previous meta-analysis. Our study included a total of eight high-quality RCTs with 670 patients, as opposed to the previous meta-analysis. In addition, the present study showed that OC was associated with a significantly reduced incidence of VAP, which no previous meta-analysis has reported before. The lung and immune system of VLBW infants are immature, and a longer mechanical ventilation time will lead to higher VAP incidence, followed by higher mortality and a longer hospital stay. In consideration of this population of premature infants, early prevention with OC is very effective. This was also the main focus of our meta-analysis. We paid more attention to whether the routine use of OC therapy could protect the lungs of premature infants with mechanical ventilation from VAP.
There are several limitations of the present meta-analysis. First, only three articles mentioned VAP, although the results indicated statistical significance, the strengths of oral colostrum for VAP still require more attention, and more RCTs are required to confirm this view further. Second, three studies produced bar charts presenting the levels of IgA and lactoferrin in serum and urine, whereas these studies were not analyzed because there were no original data. Obviously, large RCTs are required to address the potential immunological benefits of OC.
Conclusions
According to the results of the pooled analysis of the currently available data, OC indeed significantly reduced the frequency of VAP and full enteral feeding days in the NICU. Furthermore, OC is safe and feasible; the therapy process displays no side effects and does not require considerable costs. Overall, OC therapy is considered a cost-effective method for VLBW infants, and it is worthy of routine clinical application.
References
Harrison, M. S. & Goldenberg, R. L. Global burden of prematurity. Semin. Fetal Neonatal. Med. 21, 74–79 (2016).
Apisarnthanarak, A., Holzmann-Pazgal, G., Hamvas, A., Olsen, M. A. & Fraser, V. J. Ventilator-associated pneumonia in extremely preterm neonates in a neonatal intensive care unit: characteristics, risk factors, and outcomes. Pediatrics 112, 1283–1289 (2003).
Cernada, M., Brugada, M., Golombek, S. & Vento, M. Ventilator-associated pneumonia in neonatal patients: an update. Neonatology 105, 98–107 (2014).
Venkatachalam, V., Hendley, J. O. & Willson, D. F. The diagnostic dilemma of ventilator-associated pneumonia in critically ill children. Pediatr. Crit. Care Med. 12, 286–296 (2011).
Bigham, M. T. et al. Ventilator-associated pneumonia in the pediatric intensive care unit: characterizing the problem and implementing a sustainable solution. J. Pediatr. 154, 582–587 (2009). e582.
Elward, A. M., Warren, D. K. & Fraser, V. J. Ventilator-associated pneumonia in pediatric intensive care unit patients: risk factors and outcomes. Pediatrics 109, 758–764 (2002).
Tan, B. et al. Risk factors for ventilator-associated pneumonia in the neonatal intensive care unit: a meta-analysis of observational studies. Eur. J. Pediatr. 173, 427–434 (2014).
Lee, P. L., Lee, W. T. & Chen, H. L. Ventilator-associated pneumonia in low birth weight neonates at a neonatal intensive care unit: a retrospective observational study. Pediatr. Neonatol. 58, 16–21 (2017).
MathewsLM., M. Reducing the risk of ventilator-associated infectious. Dimens. Crit. Care Nurs. 19, l7–l21 (2000).
Bharwani, S. K. et al. Systematic review and meta-analysis of human milk intake and retinopathy of prematurity: a significant update. J. Perinatol. 36, 913–920 (2016).
Patel, A. L. et al. Impact of early human milk on sepsis and health-care costs in very low birth weight infants. J. Perinatol. 33, 514–519 (2013).
Klarin, B., Molin, G., Jeppsson, B. & Larsson, A. Use of the probiotic Lactobacillus plantarum 299 to reduce pathogenic bacteria in the oropharynx of intubated patients: a randomised controlled open pilot study. Crit. Care 12, R136 (2008).
Martin, R. et al. Probiotic potential of 3 Lactobacilli strains isolated from breast milk. J. Hum. Lact. 21, 8–17 (2005). quiz 18-21, 41.
Mihatsch, W. A. et al. Critical systematic review of the level of evidence for routine use of probiotics for reduction of mortality and prevention of necrotizing enterocolitis and sepsis in preterm infants. Clin. Nutr. 31, 6–15 (2012).
Thibeau, S. & Boudreaux, C. Exploring the use of mothers’ own milk as oral care for mechanically ventilated very low-birth-weight preterm infants. Adv. Neonatal Care 13, 190–197 (2013).
Rodriguez, N. A. et al. A pilot study to determine the safety and feasibility of oropharyngeal administration of own mother’s colostrum to extremely low-birth-weight infants. Adv. Neonatal Care 10, 206–212 (2010).
Gephart, S. M. & Weller, M. Colostrum as oral immune therapy to promote neonatal health. Adv. Neonatal Care 14, 44–51 (2014).
Garofalo, N. A. & Caplan, M. S. Oropharyngeal mother’s milk: state of the science and influence on necrotizing enterocolitis. Clin. Perinatol. 46, 77–88 (2019).
Seigel, J. K. et al. Early administration of oropharyngeal colostrum to extremely low birth weight infants. Breastfeed. Med. 8, 491–495 (2013).
Snyder, R. et al. Early provision of oropharyngeal colostrum leads to sustained breast milk feedings in preterm infants. Pediatr. Neonatol. 58, 534–540 (2017).
Moreno-Fernandez, J. et al. Enhancement of immune response mediated by oropharyngeal colostrum administration in preterm neonates. Pediatr. Allergy Immunol. 30, 234–241 (2019).
Abd-Elgawad, M., Eldegla, H., Khashaba, M. & Nasef, N. Oropharyngeal administration of mother’s milk prior to gavage feeding in preterm infants: a pilot randomized control trial. JPEN J. Parenter. Enteral Nutr. 44, 92–104 (2020).
Ferreira, D. M. L. M. et al. Randomized controlled trial of oropharyngeal colostrum administration in very-low-birth-weight preterm infants. J. Pediatr. Gastroenterol. Nutr. 69, 126–130 (2019).
Zhang, Y., Ji, F., Hu, X., Cao, Y. & Latour, J. M. Oropharyngeal colostrum administration in very low birth weight infants: a randomized controlled trial. Pediatr. Crit. Care Med. 18, 869–875 (2017).
Sharma, D., Kaur, A., Farahbakhsh, N. & Agarwal, S. Role of oropharyngeal administration of colostrum in very-low-birth-weight infants for reducing necrotizing enterocolitis: a randomized controlled trial. Am. J. Perinatol. (2019). https://doi.org/10.1055/s-0039-1688817.
Romano-Keeler, J. et al. Oral colostrum priming shortens hospitalization without changing the immunomicrobial milieu. J. Perinatol. 37, 36–41 (2017).
Lee, J. et al. Oropharyngeal colostrum administration in extremely premature infants: an RCT. Pediatrics 135, e357–e366 (2015).
Glass, K. M., Greecher, C. P. & Doheny, K. K. Oropharyngeal administration of colostrum increases salivary secretory IgA levels in very low-birth-weight infants. Am. J. Perinatol. 34, 1389–1395 (2017).
Rodriguez, N. A. A randomized controlled trial of the oropharyngeal administration of mother’s colostrum to extremely low birth weight infants in the first days of life. Neonatal Intens. Care 24, 31–35 (2011).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8, 336–341 (2010).
Higgins, J. P. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928 (2011).
Higgins, J. P. T. S., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Levy, M. M. et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 31, 1250–1256 (2003).
Sherman, M. P., Miller, M. M., Sherman, J. & Niklas, V. Lactoferrin and necrotizing enterocolitis. Curr. Opin. Pediatr. 26, 146–150 (2014).
Juhl, S. M. Necrotizing enterocolitis—classification and two initial steps towards prevention. Dan. Med. J. 64 (2017).
Gopalakrishna, K. P. et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat. Med. 25, 1110–1115 (2019).
Zeng, J. et al. Effect of probiotics on the incidence of ventilator-associated pneumonia in critically ill patients: a randomized controlled multicenter trial. Intens. Care Med. 42, 1018–1028 (2016).
Ohlsson, A. & Lacy, J. B. Intravenous immunoglobulin for preventing infection in preterm and/or low birth weight infants. Cochrane Database Syst. Rev. 1, CD000361 (2013).
Garg, B. D., Balasubramanian, H., Kabra, N. S. & Bansal, A. Effect of oropharyngeal colostrum therapy in the prevention of necrotising enterocolitis among very low birthweight neonates: a meta-analysis of randomised controlled trials. J. Hum. Nutr. Diet. 31, 612–624 (2018).
Panchal, H., Athalye-Jape, G. & Patole, S. Oropharyngeal colostrum for preterm infants: a systematic review and meta-analysis. Adv. Nutr. 10, 1152–1162 (2019).
Author information
Authors and Affiliations
Contributions
A.M. contributed to the conception and drafted the article. J.Y. and Y.L. revised it critically for important intellectual content. Y.K. approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
This meta-analysis was based on published studies. Therefore, the authors have no ethical conflicts to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Ma, A., Yang, J., Li, Y. et al. Oropharyngeal colostrum therapy reduces the incidence of ventilator-associated pneumonia in very low birth weight infants: a systematic review and meta-analysis. Pediatr Res 89, 54–62 (2021). https://doi.org/10.1038/s41390-020-0854-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0854-1
This article is cited by
-
The effect of oropharyngeal mother’s milk on nutritional outcomes in preterm infants: a randomized controlled trial
BMC Pediatrics (2024)
-
A quality improvement initiative to reduce necrotizing enterocolitis in high-risk neonates
Journal of Perinatology (2023)
-
A randomized controlled trial of oropharyngeal therapy with mother’s own milk for premature infants
Journal of Perinatology (2023)
-
Liquid gold: do we need to fraction fresh colostrum for oral immunotherapy in premature infants?
International Breastfeeding Journal (2022)
-
Increasing early exposure to mother’s own milk in premature newborns
Journal of Perinatology (2022)