Abstract
Background
Volatile organic compounds (VOCs) are hydrocarbons that originate within different healthy and diseased tissues. VOCs can be secreted into the circulation and then excreted in the urine and faeces. In the lungs, VOCs are locally produced and can be detected in exhaled breath. VOCs can be identified using non-invasive techniques, which make their use in preterm infants safe and desirable.
Methods
A systematic search of the literature in PubMed, Embase and Web of Science was conducted looking for VOCs techniques and diagnostic performance in preterm infants. A total of 50 articles identified with only seven papers were included in the final analysis in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Results
VOCs could diagnose necrotising enterocolitis up to 4 days before a clinical diagnosis; for late onset sepsis, up to 3 days before; and for bronchopulmonary dysplasia, up to 2 weeks before. In addition to these diagnostic uses, VOCs analysis could also distinguish breastfed from formula-fed preterm neonates in the first month of life.
Conclusion
VOCs analysis is a non-invasive tool that makes the use in preterm infants of preference. VOCs analytic techniques require more research and consensus between researchers to overcome their limitations.
Impact
-
Volatile organic compounds are hydrocarbons that can separate between healthy and diseased states in preterm infants.
-
Biomarker panels developed from volatile organic compounds are potential diagnostic tools.
-
The non-invasive nature of acquiring volatile organic compounds markers make it desirable in the paediatric patients.
-
Research into exact chemical components of the volatile organic compounds can inform about the pathophysiology of disease in preterm infants.
-
More robust longitudinal studies with repeated experiments are required before volatile organic compounds can be applied in clinical practice.
Similar content being viewed by others
Introduction
Volatile organic compounds (VOCs) are carbon-based compounds that can be captured in gaseous form at room temperature. VOCs can be detected in bodily secretions like sweat, urine, faeces, and exhaled breath.1 VOCs are an outcome of the cellular metabolic functions; hence, VOCs production vary when the cell undergoes differentiation and division in different disease processes.1 Techniques used to detect VOCs include the operation of gas chromatography (GC). It aims to identify the different signature or patterns of VOCs. These patterns are not single compounds but rather fusions of them. VOCs patterns could be identified using electronic devices that employ GC like ‘electronic nose’ (eNose).2 Coupling GC technique to mass spectrometry (GC-MS) could help to identify the exact chemical composition of each VOC. Examples of devices that include GC-MS techniques include field asymmetric ion mobility spectrometry (FAIMS) and selected ion flow tube mass spectrometry (SIFT-MS). These devices have the potential to identify underlying pathological processes, by determining the VOCs composition produced by diseased tissues, and hence it could be used diagnostically.
Recent research supporting the use of VOCs in the diagnosis of diseases such as inflammatory bowel disease,3 malignancies,4 and even infections like tuberculosis5 in adults. Indeed, more recent studies have also indicated the usefulness of VOCs as diagnostic tools in paediatrics, particularly for gastrointestinal diseases,6 asthma and infectious exacerbations of cystic fibrosis.7 Due to the non-invasive nature of testing for VOCs, they are a desirable tool for use in paediatrics and neonatology, as they cause less distress than current investigative techniques, as well as being rapid and having the potential to give ‘point of care’ diagnoses.
Preterm infants are highly susceptible to disease and infection, as they are born without the fully developed adaptations to extrauterine life that infants born at term possess. Without the production of surfactant in the lungs, preterm infants are susceptible to developing respiratory distress of the newborn and requiring invasive ventilation, which then predisposes them to bronchopulmonary dysplasia (BPD) and chronic lung disease. An immature gut microbiome, which differs in composition to that of healthy term infants,8 puts preterm infants at risk of developing necrotising enterocolitis (NEC), gut perforation and gastrointestinal infections, which then may progress into a late onset sepsis (LOS).9 In some cases, infants are born too premature to be able to feed enterally and must be parenterally fed through a central line, predisposing them to further infection and preventing development of a normal gut microbiome. Clearly this is a naturally vulnerable patient group, with mortality rates increasing with increasing prematurity.10 Mortality rates are estimated to be between 11 and 19% for neonatal sepsis,9 and between 20 and 30% for necrotising enterocolitis, depending on birth weight;11 therefore, early detection and treatment is beneficial.
Currently there is no universal definition of clinical neonatal sepsis,12 and diagnosis may involve blood and urine cultures, cerebrospinal fluid examination, or measuring systemic inflammatory markers like C-reactive protein.13 Current diagnosis of necrotising enterocolitis involves meeting certain criteria (either Bell’s or the adapted version by Walsh and Kliegman), using clinical and radiological findings.14 Bronchopulmonary dysplasia is generally diagnosed when an infant requires oxygen therapy at certain ages, usually a continued requirement beyond 28 days of life, but is not currently diagnosed in advance of this.15
The aim of this systematic review is to identify the existing literature on the use of VOCs testing in preterm infants, and to determine if this would improve the speed of diagnosis of important illnesses in this vulnerable patient group.
Materials and methods
Search strategy
A systematic literature search was conducted in January 2020, in the PubMed, Embase and Web of Science databases. The final search terms used were VOCs OR (‘volatile organic compounds’ [MeSH Terms] OR (‘volatile’ AND ‘organic’ AND ‘compounds’) OR ‘volatile organic compounds’) AND (‘infant, premature’ [MeSH Terms] OR (‘infant’ AND ‘premature’) OR ‘premature infant’ OR (‘preterm’ AND ‘infants’) OR ‘preterm infants’) in PubMed, and kept as similar as possible in other databases. Papers were limited to those in English. Results were screened for duplicates manually, then the full articles were assessed for eligibility using the inclusion and exclusion criteria outlined below.
Inclusion criteria
Studies included were those focusing on preterm infants, defined as neonates born before 37 weeks gestation. To be included, studies also had to have a focus of using VOCs diagnostically.
Exclusion criteria
Studies were excluded if the subjects were born later than 37 weeks gestation, and if they were focusing on the effects of inhaling or consuming VOCs, rather than analysing them for diagnostic purposes. Literature reviews, conference proceedings and abstracts were also excluded.
Study selection
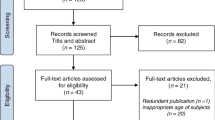
The search collected a total of 50 articles: 18 in PubMed, 11 in Embase and 21 in Web of Science. After duplicates were removed, 28 articles remained, and screening in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) resulted in 21 requiring assessment of eligibility using the exclusion and inclusion criteria outlined above. Seven papers were then included in the final analysis (Fig. 1).
Study characteristics
For each study, PICO (population, intervention, comparator, outcome) was extracted (Table 1). VOCs analysis was generally used as the intervention, using either an eNose, GC-MS or FAIMS technology depending on the study. This was compared to clinical diagnoses of the diseases, such as using the Vermont criteria for LOS,16,17 or either the international classification of Walsh and Kliegman18 or Bell’s criteria for necrotising enterocolitis.
Main outcome
The main outcome of the review was to measure number of days before a clinical diagnosis infants with the disease could be discriminated from controls using VOCs analysis. In this review, the clinical significance is defined as a diagnosis made 12 or more hours earlier than standard diagnostic or clinical measures.
Results
Preclinical diagnosis of necrotising enterocolitis
Three studies obtained results concerning the preclinical diagnosis of NEC. de Meij et al.18 found that the VOCs smell print differed between infants with the disease and healthy matched controls up to 3 days before the clinical diagnosis was apparent. Another case control study identified the absence of four specific esters, namely 2-ethylhexyl acetic ester, decanoic acid ethyl ester, dodecanoic acid ethyl ester, and hexadecenoic acid ethyl ester from the NEC group 4 days before the infants developed the disease.19 A recent study by Probert et al. was a negative study with odds ratio ranging from 0.5 to 0.7 and did not show clear correlation between NEC and faecal VOCs. The study concluded that the data were insufficient to enable reliable cotside detection of babies at risk of developing NEC.20
Preclinical diagnosis of respiratory diseases
One study examined the diagnosis of BPD using VOCs, while one studied whether congenital pneumonia could be distinguished from congenital diaphragmatic hernia using VOC analysis. Both faecal samples and exhaled breath condensate (EBC) were used for VOCs analysis of respiratory diseases. Infants that developed BPD could be distinguished from healthy controls at 14, 21 and 28 days postnatally,21 while the diagnosis of BPD can usually only be made clinically after 28 days of oxygen dependency. Intubated infants with congenital pneumonia could be distinguished from controls with left-sided congenital diaphragmatic hernia based on the VOCs given off from their exhaled breath condensate22 (although this was not tested before clinical onset), demonstrating that VOCs analysis has the potential to distinguish between infectious and non-infectious diseases.
Preclinical diagnosis of late onset sepsis
The two studies that investigated the diagnosis of LOS both analysed the VOCs of daily faecal samples produced by neonates born before 30 weeks’ gestation, from day 3 after birth. They found that the diagnosis using VOCs analysis of faecal samples could be made up to 3 days before a clinical diagnosis.16,17 Indeed, for sepsis caused by Escherichia coli or Staphylococcus aureus, the diagnosis could be made very accurately.17 Furthermore, the VOCs were not tested prior to 3 days before clinical onset, and so there is the potential that the diagnosis could be made even earlier than this.
Discussion
The findings summarised in this systemic review go far beyond the initial studies using exhaled breath VOCs to detect respiratory conditions and faecal VOCs to diagnose gastrointestinal diseases and suggest that VOCs have widespread diagnostic applications. It therefore follows that these developments could also be implemented for diagnostic use in the preterm infant patient group. The limited selection of studies so far supports the hypothesis that VOC analysis could provide an earlier diagnosis of diseases such as necrotising enterocolitis, LOS and BPD, than the methods and clinical criteria currently in place. Specifically, findings show that VOCs analysis may enable diagnosis of NEC up to 4 days before clinically apparent,18,19 diagnosis of LOS up to 3 days prior to clinically,16,17 and diagnosis of BPD up to 14 days before it can be clinically established.21
Life-threatening illnesses in preterm infants are to some degree unavoidable due their immaturity in development; however, some complications should be preventable, with improved diagnostic methods and adaptations in care.23,24 Most diagnostic measures used in this patient group are currently invasive, such as taking blood cultures and lumbar puncture to detect sepsis.12 These methods not only are a potential source of infection but also may induce stress and pain in the neonates, which has the potential to affect cognitive and behavioural development in the long term.25 Even the recent suggestion to use ultrasound scans to diagnose necrotising enterocolitis26 is likely to cause more stress to the developing neonate than VOCs analysis, as it would involve direct contact with the skin. Early intervention is essential to prevent further complications, such as multiple organ impairment in necrotising enterocolitis, and death. We therefore suggest that there is a need for new and improved diagnostic measures, both to avoid invasive testing and for earlier diagnosis to enable early intervention.
VOCs analysis is a developing area of diagnostics, with an increasing research base. Further work would need to be carried out in order to bring this measure into clinical practice as an accurate and reliable diagnostic tool, independent of clinical, radiological and biochemical investigations. Research into this area is gaining momentum, with recent papers exploring the possibility of using the eNose to diagnose polycystic ovary syndrome,27 recurrent head and neck cancer,28 and diabetes,29 among many others. One finding that could be particularly useful diagnostically is the point that the eNose could accurately detect when LOS was caused by E. coli or S. aureus.17 The possibility of preclinically diagnosing not only sepsis, but also the organism causing it, is highly desirable as this would enable the initiation of narrow spectrum, targeted antibiotics much earlier than otherwise. If VOC analysis could be used to identify the causative organism, this would reduce the need for blood culture samples, providing a less invasive diagnostic option, while also saving time in terms of earlier diagnosis and no laboratory analysis time.
Choice of VOCs source
This review demonstrates that faecal samples are the most extensively studied source of VOCs in this patient group. A number of the studies originate from the same research group, using a technique they have established for VOCs extraction. Practically, faecal samples are relatively simple to obtain and store compared to breath or urinary samples, as they can be easily removed from nappies and frozen until analysis is required. However, difficulties in using this method were reported, including the lack of regular samples available when infants did not necessarily defaecate every day. Infants receiving total parenteral nutrition may defaecate infrequently as little matter passes through their bowels, and therefore faecal sampling may not always be achievable on a regular basis.
One study instead uses EBC as a VOC source.22 Other studies outside of this review have investigated the analysis of EBC in preterm infants in identifying inflammatory states and neonatal chronic lung disease.30 Exhaled breath analysis has already been demonstrated to be a potential diagnostic tool within other areas of paediatrics6 and in adults with tuberculosis for example.31 Arguably these findings could be extended to preterm infants, with EBC having the advantage over faecal samples of being constantly available, rather than variable in production. For the diagnosis of diseases like sepsis, where every hour impacts on survival, a constant source of VOCs may be more appropriate than a daily source. However, the practicality of extracting EBC from preterm neonates is more complicated than for faecal matter. It either requires the infants to be intubated (which should be avoided if not necessary), or the development of an eNose device that attaches within incubators and monitors the VOC profiles of exhaled breath within the patient’s environment. A recent study has demonstrated the possibility of bedside measurement of VOCs in the atmosphere of neonatal incubators, by finding that occupied incubators could be distinguished from unoccupied incubators using VOC analysis.32 Analysis of VOCs in EBC could provide an earlier diagnosis of disease than faecal samples, as exhaled breath could be collected at any time, and multiple times a day.
Few studies have investigated the use of urinary samples as a source of VOCs in this patient group. In adults, urinary VOC analysis has been shown to have potential in diagnosing renal disease,33,34 liver disease,35 pancreatic cancer36 and even colorectal cancer.37,38 However, one study reported the practical difficulty of procuring urinary samples from neonates, including contamination with faecal matter within the nappies containing the samples.39 Therefore, although urine could be a valid source of VOCs, it may not be practically realistic in this patient group without developing alternative methods of sample collection. Other sources of VOCs are yet to be investigated in this patient group. However, there is likely to be little scope for other sources, such as sweat, in these patients, due to their physiological immaturity.
Limitations
One confounding variable to consider is the idea that the environment and nutrition newborns are exposed to may affect the spectrum of VOCs they produce. For example, one study has explored the effect of enteral feeding composition on faecal VOCs in preterm infants.40 It was found that there was a statistically significant difference between the VOC profiles of preterm infants fed primarily with breast milk and those fed primarily with formula milk when profiles from 7, 14 and 21 days postnatally were combined. This demonstrates therefore that factors other than disease processes may influence VOC composition, and an awareness of this should be present when attempting to use VOCs as a diagnostic tool.
Conclusion
VOCs could diagnose necrotising enterocolitis and LOS 3−4 days before a clinical diagnosis is made and up to 2 weeks before a diagnosis for BPD is reached. VOCs analysis could also distinguish breastfed from formula-fed preterm neonates in the first month of life. VOCs hold potential as non-invasive diagnostic tools and in certain conditions afflicting preterm infants, enable earlier diagnosis with improved outcomes. However, more research is needed in the paediatric group with more properly designed prospective and longitudinal studies to establish the use of VOCs analysis as a diagnostic technique.
References
Shirasu, M. & Touhara, K. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J. Biochem. 150, 257–266 (2011).
Padua, A. C., Palma, S., Gruber, J., Gamboa, H. & Roque, A. C. Design and evolution of an opto-electronic device for VOCs detection. Biomed. Eng. Syst. Technol. Int. Jt. Conf. BIOSTEC Revis. Sel. Pap. 1, 48–55 (2018).
Arasaradnam, R. P. et al. Non-invasive exhaled volatile organic biomarker analysis to detect inflammatory bowel disease (IBD). Dig. Liver Dis. 48, 148–153 (2016).
Sun, X., Shao, K. & Wang, T. Detection of volatile organic compounds (VOCs) from exhaled breath as noninvasive methods for cancer diagnosis. Anal. Bioanal. Chem. 408, 2759–2780 (2016).
Zetola, N. M. et al. Diagnosis of pulmonary tuberculosis and assessment of treatment response through analyses of volatile compound patterns in exhaled breath samples. J. Infect. 74, 367–376 (2017).
Buijck, M. et al. Sniffing out paediatric gastrointestinal diseases: the potential of volatile organic compounds as biomarkers for disease. J. Pediatr. Gastroenterol. Nutr. 63, 585–591 (2016).
Bannier, M., van de Kant, K. D. G., Jobsis, Q. & Dompeling, E. Feasibility and diagnostic accuracy of an electronic nose in children with asthma and cystic fibrosis. J. Breath Res. 13, 036009 (2018).
Tauchi, H. et al. Gut microbiota development of preterm infants hospitalised in intensive care units. Benef. Microbes 9, 1–12 (2019).
Fleischmann-Struzek, C. et al. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir. Med. 6, 223–230 (2018).
Walsh, E. M., Li, S. X., Black, L. K. & Kuzniewicz, M. Incremental cost of prematurity by week of gestational age. AJP Rep. 9, 76–83 (2019).
Fitzgibbons, S. C. et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J. Pediatr. Surg. 44, 1072–1075 (2009).
Chiesa, C., Panero, A., Osborn, J. F., Simonetti, A. F. & Pacifico, L. Diagnosis of neonatal sepsis: a clinical and laboratory challenge. Clin. Chem. 50, 279–287 (2004).
Beltempo, M. et al. C-reactive protein for late-onset sepsis diagnosis in very low birth weight infants. BMC Pediatr. 18, 1–8 (2018).
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729 (2001).
Berkhout, D. J. C. et al. Detection of sepsis in preterm infants by fecal volatile organic compounds analysis: a proof of principle study. J. Pediatr. Gastroenterol. Nutr. 65, e47–e52 (2017).
Berkhout, D. J. C. et al. Late-onset sepsis in preterm infants can be detected preclinically by fecal volatile organic compound analysis: a prospective, multicenter cohort study. Clin. Infect. Dis. 68, 70–77 (2019).
de Meij, T. G. J. et al. Early detection of necrotizing enterocolitis by fecal volatile organic compounds analysis. J. Pediatrics. 167, 562–567 (2015).
Garner, C. E. et al. Analysis of faecal volatile organic compounds in preterm infants who develop necrotising enterocolitis: a pilot study. J. Pediatr. Gastroenterol. Nutr. 49, 559–565 (2009).
Probert, C. et al. Faecal volatile organic compounds in preterm babies at risk of necrotising enterocolitis: the DOVE study. Arch. Dis. Child. Fetal Neonatal Ed. Dec 23 (2019). [Epub ahead of print].
Berkhout, D. J. C. et al. Development of severe bronchopulmonary dysplasia is associated with alterations in fecal volatile organic compounds. Pediatr. Res. 83, 412–419 (2018).
Kononikhin, A. S. et al. Exhaled breath condensate analysis from intubated newborns by nano-HPLC coupled to high resolution MS. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 1047, 97–105 (2017).
Embleton, N. D. et al. Mechanisms affecting the gut of preterm infants in enteral feeding trials. Front. Nutr. 4, 1–13 (2017).
Park, J. H., Chang, Y. S., Sung, S. & Park, W. S. Mortality rate-dependent variations in the timing and causes of death in extremely preterm infants born at 23–24 weeks’ gestation. Pediatr. Crit. Care Med. 20, 630–637 (2019).
Vinall, J. & Grunau, R. E. Impact of repeated procedural pain-related stress in infants born very preterm. Pediatr. Res. 75, 584–587 (2014).
Deeg, K. H. Sonographic and Doppler sonographic diagnosis of necrotizing enterocolitis in preterm infants and newborns. Ultraschall. Med. 40, 292–318 (2019).
Sankarganesh, D. et al. Urinary volatile metabolomics as a viable alternative diagnostic tool for polycystic ovary syndrome: an exploratory hypothesis. Med. Hypotheses 124, 121–124 (2019).
van de Goor, R., Hardy, J. C. A., van Hooren, M. R. A., Kremer, B. & Kross, K. W. Detecting recurrent head and neck cancer using electronic nose technology: a feasibility study. Head Neck 41, 2983–2990 (2019).
Behera, B., Joshi, R., Anil Vishnu, G. K., Bhalerao, S. & Pandya, H. J. Electronic nose: a non-invasive technology for breath analysis of diabetes and lung cancer patients. J. Breath Res. 13, 024001 (2019).
Harrison, C. M. & Andersen, C. C. Exhaled breath measures of inflammation: are they useful in neonatal chronic lung disease. Arch. Dis. Child. Fetal Neonatal Ed. 90, 6–10 (2005).
Sahota, A. S. et al. A simple breath test for tuberculosis using ion mobility: a pilot study. Tuberculosis (Edinburgh) 99, 143–146 (2016).
Steinbach, J. et al. Bedside measurement of volatile organic compounds in the atmosphere of neonatal incubators using ion mobility spectrometry. Front. Pediatrics 7, 1–7 (2019).
Liu, D. et al. Urine volatile organic compounds as biomarkers for minimal change type nephrotic syndrome. Biochem. Biophys. Res. Commun. 496, 58–63 (2018).
Wang, M., Xie, R., Jia, X. & Liu, R. Urinary volatile organic compounds as potential biomarkers in idiopathic membranous nephropathy. Med. Princ. Pract. 26, 375–380 (2017).
Arasaradnam, R. P. et al. Non-invasive distinction of non-alcoholic fatty liver disease using urinary volatile organic compound analysis: early results. J. Gastrointestin Liver Dis. 24, 197–201 (2015).
Arasaradnam, R. P. et al. Noninvasive diagnosis of pancreatic cancer through detection of volatile organic compounds in urine. Gastroenterology 154, 485–487 (2018).
Widlak, M. M. et al. Risk stratification of symptomatic patients suspected of colorectal cancer using faecal and urinary markers. Colorectal Dis. 20, 335–342 (2018).
Arasaradnam, R. P. et al. Detection of colorectal cancer (CRC) by urinary volatile organic compound analysis. PLoS ONE 9, 1–9 (2014).
El-Metwally, D. et al. Urinary metabolites of volatile organic compounds of infants in the neonatal intensive care unit. Pediatr. Res. 83, 1158–1164 (2018).
El Manouni El Hassani, S. et al. Fecal volatile organic compounds in preterm infants are influenced by enteral feeding composition. Sensors (Basel) 18, 1–13 (2018).
Author information
Authors and Affiliations
Contributions
H.W. collected and analysed the data and participated in the final manuscript. A.S.B., R.I. and M.M. contributed to all aspects of the final manuscript for analysis and intellectual input. R.P. A. designed the study and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wright, H., Bannaga, A.S., Iriarte, R. et al. Utility of volatile organic compounds as a diagnostic tool in preterm infants. Pediatr Res 89, 263–268 (2021). https://doi.org/10.1038/s41390-020-0828-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0828-3