Abstract
Background
The inefficiency of recording data repeatedly limits the number of studies conducted. Here we illustrate the wider use of data captured as part of the European eNewborn benchmarking programme.
Methods
We extracted data on 39,529 live-births from 22 weeks 0 days to 31 weeks 6 days gestational age (GA) or ≤1500 g birth weight. We explored relationships between delivery room care and Apgar scores on mortality and bronchopulmonary dysplasia (BPD) and calculated the time needed for each country to detect a clinically relevant change in these outcomes following a hypothetical intervention.
Results
Early neonatal, neonatal, and in-hospital mortality were 3.90% (95% CI 3.71, 4.09), 6.00% (5.77, 6.24) and 7.57% (7.31, 7.83), respectively. The odds of death were greater with decreasing GA, lower Apgar scores, growth restriction, male sex, multiple birth and no antenatal steroids. Relationships for BPD were similar. The time required for participating countries to achieve 80% power to detect a relevant change in outcomes following a hypothetical intervention in 23–25 weeks’ GA infants ranged from 12 years for neonatal mortality and 22 years for BPD compared to 1 year for the whole network.
Conclusions
The eNewborn platform offers opportunity to drive efficiencies in benchmarking, quality control and research.
Similar content being viewed by others
Introduction
Very preterm, very low birth weight infants are major contributors to the population burden of mortality and morbidity related to perinatal factors; hence, efforts to improve the quality of care for this patient group is an important area of health service endeavour.1 Data collection and analyses of processes and outcomes across neonatal units and networks are potentially informative and can be powerful tools to improve quality by benchmarking and feedback. Such data might also offer substantial potential for research.
There are, however, many practical challenges involved in utilising data from multiple countries to improve neonatal care. There are differences between and within countries in the methods used to record data, the regulatory approvals governing their use, consistency in the items recorded and their definitions, no less in clinical practice.2 These difficulties can be major obstacles to realising their potential. In Europe, the open platform EuroNeoNet, was an initial attempt to record and utilise data from neonatal units.3 EuroNeoNet closed in 2015 and was followed by eNewborn, a platform integrating innovative information technology, original software, a revision of the EuroNeoNet data set, and international collaboration. The characteristics of the eNewborn platform have been described elsewhere.4
The aim of this paper is to report a pilot evaluation of eNewborn as a large, real-world data set. We used selected variables and an exemplar statistical analysis to provide an indication of the potential utility of the eNewborn database. Our specific objectives were to use the eNewborn database to (i) present summary statistics for early, neonatal and in-hospital mortality by gestational age (GA); (ii) analyse the relationships between delivery room care and Apgar scores at 1 and 5 min on mortality and bronchopulmonary dysplasia (BPD) as a first step for the development of a future tool to predict risks of these outcomes in extremely and very low birth weight infants; and (iii) calculate the length of time that would be needed by individual countries on the basis of their neonatal admission rates to achieve 80% power to detect a clinically relevant change in mortality and BPD following a hypothetical intervention.
Methods
Data source
The eNewborn database receives information on all live births born between 22 weeks 0 days and 31 weeks 6 days of gestation or ≤1500 g birth weight who are admitted to a participating neonatal unit. Data on babies who die in the delivery room are not included as information on these infants varies within and between countries. Over 200 neonatal units across Europe submit data to eNewborn. Belgium, Czech Republic, Portugal, Switzerland and the United Kingdom submit data extracted from an in-country database. Data are submitted by individual neonatal units from 5 additional countries: France 10 units, Germany 1 unit, Poland 1unit, Spain 2 units, and Finland 1 unit. Data entry was either directly online or as an extract from local electronic medical records. The proportional contribution of each country is described in Appendix 1.
Definitions
We defined mortality as ‘early’ (days 0–6 from birth), ‘neonatal’ (days 0–27) and ‘in-hospital’ (before discharge from neonatal care) and BPD as any supplemental oxygen received at 36 weeks’ postmenstrual age. We defined delivery room care as ‘no resuscitation’, ‘basic resuscitation’ (supplemental oxygen and/or positive pressure breaths with a bag and face mask or continuous positive airway pressure)’ and ‘advanced resuscitation’ (endotracheal intubation and/or cardiac compression and/or administration of adrenaline/epinephrine). We categorised birth weight as extremely small for gestational age (ESGA; <3rd centile), small for gestational age (SGA; <10th centile) and appropriate for gestational age (AGA; ≥10th centile).5
Statistical analysis
We extracted 12 variables for each infant: (i) GA; (ii) birth weight; (iii) delivery room care (none, basic, advanced); (iv) Apgar at 1 min; (v) Apgar at 5 min; (vi) BPD (yes/no); (vii) death (early, neonatal, in-hospital; none); (viii) antenatal steroids (yes/no/partial); (ix) multiple birth (yes/no); (x) inborn/outborn; (xi) surfactant yes/no; (xii) sex. For each variable, we tabulated the number of infants and the number of missing values. As birth weights <250 g are implausible for liveborn infants, we classified these as missing. Major birth defects were recorded.
We estimated early neonatal, neonatal and in-hospital mortality for boys and girls combined and separately. To investigate the relationship between delivery room care and 1 and 5 min Apgar scores, we first explored their correlations by GA. We examined the evolution of Apgar scores by fitting an independence model to determine whether an association between 1 and 5 min values could be demonstrated. In case of a significant association, we tested the symmetry of the changes in these variables, presented as a square table, by Bowker’s test.6 In case of a significant test, we used the off-diagonal elements of the table to judge the direction in which the changes occurred. After the independence model, we fitted a model of linear by linear association to examine the effect of antenatal steroids on the Apgar score at 1 min. A finding of increasing Apgar scores with increasing compliance with antenatal steroid administration would reject the hypothesis of no association.6
We then conducted four exploratory logistic analyses. In the first analysis, we fitted ‘neonatal mortality’ as the endpoint against delivery room care category, GA, birth weight category, antenatal steroids, multiple birth and sex. To avoid problems of multicollinearity, we modelled birth weight category rather than birth weight. In the second analysis, we replaced delivery room care category with Apgar score at 1 min and in the third with Apgar score at 5 min. In addition, we fitted a similar model with the evolution of the Apgar score from minute 1 to minute 5, replacing delivery room care, Apgar scores at 1 min and at 5 min of the previous models. Similarly, we carried out a second series of three logistic regressions this time with BPD as the outcome and adding surfactant administration to the above explanatory variables. We had to deal with the hierarchical structure of the data consisting of infants in neonatal intensive care unit (NICU) and NICU in countries, leading to correlations within data. These correlations are contrary to the requirement of independence and may produce what is called extra-binomial variation: the variance of the dependent variable (here neonatal mortality and BPD) will be greater than expected under the assumption of a binomial distribution. This may result in underestimation of the standard errors and overestimation of the chi-square statistics. To correct for this problem, we divided all the individual chi-squares and standard errors by the ratio of the Pearson’s goodness-of-fit chi-square to its degrees of freedom, leaving the coefficient estimates unchanged.7,8
We checked model fit by the ‘c-statistic’.9 Since we were dealing with non-nested models (these are models in which no model is a subset of one of the other models), we carried out a model selection using the Bayesian Information Criterion (BIC).10 BIC is a function of the probability of a model, the number of parameters and the number of observations. It ranks the models studied according to their score, with the lower the BIC score, the better the fit.
To assess the biases that might arise from missing data, we carried out bivariate (cross-tabulation) and multivariate (logistic regression) sensitivity analyses by creating a category for missing values for Apgar scores at 1 and 5 min and antenatal steroids and compared their effect with that of non-missing values on in-hospital mortality and BPD.
To illustrate the benefits of bringing together data from different countries, we conducted a power analysis. We calculated how many years would be needed for each country to identify a statistically significant difference in mortality following a hypothetical intervention. To improve intelligibility, we reduced the 3-year period of observations to average yearly number of admissions. We provide details of the calculations in Appendix 2.
Participation in the study was agreed on according to each country’s national regulations. Agreements were network based or unit based.
Results
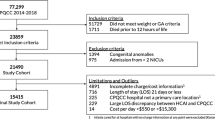
We identified 39,529 infants in the eNewborn database over the 3-year period 2014–2016, born at or below a GA of 31 weeks and 6 days or ≤1500 g birth weight. Mortality and patient characteristics are shown in Table 1. The early neonatal, neonatal and in-hospital mortality rates were 3.90% (95% confidence interval (CI) 3.71, 4.09), 6.00% (95% CI 5.77, 6.24) and 7.57% (95% CI 7.31, 7.83), respectively. Of the 2373 babies who died in the neonatal period, approximately two-thirds were in the first postnatal week. Birth defects occurred in 7.29% (n = 173), which represents 0.43% of the neonatal deaths among which 0.18% (n = 73) were related to a major congenital or chromosomal anomaly. This is a negligible contribution, and therefore we did not include congenital anomalies in the multivariate analysis.
We show early neonatal, neonatal and in-hospital mortality rates and 95% CIs by GA for boys and girls combined in Fig. 1. The decline in mortality with increasing GA is exponential up to 26 weeks and stabilises afterwards.
Neonatal mortality by sex, birth weight and GA are shown in Fig. 2. To avoid the undue effect of superimposition of cases, we plotted a random sample, defined as equal to the number of deceased newborns, of boys and girls surviving the neonatal period in the upper part of the graphs. In the lower part, we plotted the corresponding graphs for deceased boys and girls. For ease of interpretation, we added the 3rd, 10th, 90th and 97th Fenton centiles. The figure shows greater representation of deceased babies at the lower GAs and in the growth-restricted categories.
Neonatal survivors and deaths. Upper panel left: random sample of 1021 survivors (girls); Upper panel right: random sample of 1346 survivors (boys). Lower panel left: 1021 deaths (girls); Lower panel right: 1346 deaths (boys). Dashed lines from bottom to top 3rd, 10th, 90th, and 97th Fenton centile.
Table 2 describes the correlation between the three categories of delivery room management (none, basic and advanced) and Apgar score. In the smallest GA groups (22–24 weeks), we observed very weak (<0.20) correlations between Apgar scores at both 1 and 5 min and delivery room care. The correlation coefficient between Apgar score and delivery room care increased with increasing GA from 0.13 at 22–24 weeks to 0.50 >30 weeks at 1 min and from 0.09 to 0.30 at 5 min. There were strong correlations (0.60) between both Apgar scores in the most immature GA groups decreasing progressively to a moderate correlation of 0.44 in the GA group ≥30 weeks.
For babies <25 weeks GA, 95% received advanced delivery room care if the 1 min Apgar score was between 0 and 3, 92% when the 1 min Apgar was 4–6 and 82% when the 1-min Apgar was 7–10. For GAs between 25–27 and 28–31 weeks, these percentages were 90%, 80% and 56% and 69%, 40% and 14%, respectively.
Table 3 shows a significant (p < 0.001) discrepancy between the observed and expected values obtained by an independent model for both antenatal steroids and 5 min Apgar score. There was a significant improvement in Apgar score between 1 and 5 min (Bowker’s test for symmetry: p < 0.0001). Overall, 59.6% of the 36,877 newborns had the same Apgar score at 1 and 5 min, 39.3% had an improved score and 1.1% a deteriorated score (Table 2). Of the 36,877 babies, 6251 (17.0%) had a 1-min Apgar score between 0 and 3. Of these, by 5 min, 19.8% had a similar Apgar score, 47.7% had a score of 4–6 and 32.5% a score of 7–10. Of the newborns with a 1-min Apgar score of 4–6, by 5 min 80.9% had an improved and 0.6% a deteriorated score. Of those with a 1-min Apgar score of 7–10, 1.9% had a worse score at 5 min.
Increasing compliance with administration of antenatal steroids was associated with higher Apgar scores (p < 0.001).
The logistic regression modelling showed increased neonatal mortality with advanced delivery room resuscitation, Apgar scores of 0–3 and 4–6, no improvement of very low Apgar scores, decreasing GA, ESGA and SGA status, boys, multiple births and no antenatal steroids (Table 4). Babies with an incomplete course of antenatal steroids also fared worse than those with a complete course. The fit of the models was very good, all had a c-statistic between 0.84 and 0.86. According to the BIC, the ranking of the models was as follows: (1) delivery room care, (2) Apgar score at 5 min, (3) Apgar score at 1 min, and (4) evolution of the Apgar score.
In the BPD logistic regression modelling, we observed an adverse association with advanced delivery room resuscitation, Apgar scores of 0–3 and 4–6, decreasing GA, ESGA and SGA status, boys, multiple births, surfactant administration and a complete antenatal steroid course (Table 4). The fit of the models was very good; all had a c-statistic between 0.84 and 0.85. According the BIC the ranking of the models was as follows: (1) Apgar score at 5 min, (2) delivery room care, (3) Agar score at 1 min, and (4) evolution of the Apgar score.
The association between missing 1- and 5-min Apgar scores and neonatal mortality was very similar to that of the non-missing scores in both bivariate and multivariable analyses. Similarly, the effect of missing antenatal steroids was close to the ‘incomplete antenatal steroid course’ category. In the bivariate analysis, infants with a missing 1-min Apgar score had a 3.5% lower risk of BPD than the lowest 1-min Apgar score category, whereas in the multivariable analysis BPD risk was 7% higher. In the same analyses, with ‘missing antenatal steroids’ compared to non-missing antenatal steroids, we observed about 5% higher risk in the former in both the bivariate and multivariable analyses.
In Table 5, we display the number of years needed for each country or network to achieve 80% power to identify a statistically significant change in neonatal mortality and BPD in 23, 24 and 25 weeks’ GA infants. The data are presented for countries with complete data, using their ‘rates’ as present in the database and assuming these countries have similar therapeutic approaches and outcomes in terms of neonatal mortality and BPD.
Discussion
In this first analysis of the eNewborn database, we provide baseline figures for mortality and BPD for almost 40,000 very preterm/very low birth weight babies across Europe. We show that in-hospital mortality mirrors survival rates reported elsewhere11,12 with the 50% GA-specific mortality rate situated at 23–24 weeks, also in keeping with other studies.13 However, the Epice Research Group study reported an in-hospital mortality of 13.6% compared to that of our cohort, namely 7.6 %. This might be partly due to the inclusion of all babies <32 weeks or <1500 g in eNewborn with more growth-restricted mature babies increasing the survival rate.
We showed a significant increase in Apgar scores between 1 and 5 min and an association between increasing compliance with antenatal steroid administration and increased 1-min Apgar score.14 We found that, of babies with a 1-min Apgar score between 0 and 3, almost 20% had no improvement by 5 min. We further observed (1) a higher mortality for Apgar scores of 0–3 and 4–6 at 1 min and for Apgar score 0–3 at 5 min and (2) lowered mortality with improved Apgar scores at 5 min, indicating the importance of recovery in the very few first minutes of life.
We also found for babies with a 1-min Apgar score of 7–10, indicating good condition at birth, that advanced delivery room care was reported for >80% of babies at <25 weeks’ GA and over half at 25–27 weeks but was associated with high mortality and BPD. We identified as anticipated a predominant association between decreasing GA and adverse outcomes and significant associations with low Apgar scores, growth restriction, male sex and multiple birth. The odds of death were reduced across all GAs in infants who received both a complete or incomplete course of antenatal steroids. However, improved survival was accompanied by a higher risk of BPD.
The strengths of our study are the large number of infants, with <10% missing values,15 and the number of countries contributing complete or near complete neonatal unit admission data (Appendix 1). Europe has small and large countries and therefore their individual data contributions are inevitably non-homogenous. Differences in morbidity and mortality have been largely described between neonatal units and hospitals16,17,18,19,20 but less often between and within countries. We included several adjustments (GA, Apgar scores, AGA, gender, multiplets, prenatal steroids) that impact on mortality in our logistic regressions. The c-statistics, reflecting the fit of the models, are very good (all between 0.84 and 0.86) and therefore not including country as a covariate into the analysis seems not to have been a major drawback. The selection of ‘Model Delivery Room Care’ as the best performing model in the context of neonatal mortality is due to the higher number of observations, its log-likelihood of −4993.7 being smaller than that be considered of −4297.7 of ‘Model 5 min Apgar score’. In the context of BPD, ‘Model 5 min Apgar score’ performed best (see Appendix 3: model fit and model selection). Although ‘Evolution Apgar score’ is an appealing model, it remains a second-choice model due to a lesser likelihood and an increased number of parameters (combinations).
Furthermore, we limited ourselves in this exploratory phase to main effects models (models without interactions) as these are easier to understand than models with interactions. The fit of our models was such that the effect of the interactions was minimal. However, if we want to assess prediction, we must take interactions into account; for example, Fig. 2 clearly shows that there is an interaction between GA and birth weight category on neonatal mortality. Identifying optimal weightings and validating them needs sophisticated analyses and large data sets, hence will be possible in the future when the network has grown.
We also show that bias due to missing data was marginal for BPD and absent for Apgar scores. Study weaknesses are that we had no means to quality assure or cross-validate data submitted directly by individual neonatal units, incomplete population coverage and inconsistent definitions of key outcome measures, such as BPD.21 Further we have no information on socioeconomic status and other maternal details nor risk variables such as the Clinical Risk Index of Babies score.
Despite limitations, we feel some broad observations are possible. As anticipated, the most immature babies had lower Apgar scores than babies of greater maturity. Reassuringly, we identified a positive correlation between antenatal steroid exposure and 1-min Apgar score. In the most immature groups, we identified both a poor correlation between 1- and 5-min Apgar scores and delivery room care. This suggests that, across the countries represented, neonatal teams may not be necessarily intervening with advanced resuscitation attempts in the most immature infants, reflecting current thinking in relation to the care of infants at the margins of viability. The high reported rate of advanced delivery room care in babies in good condition at birth, particularly in those of greater immaturity, suggests persisting resistance to adopting a gentler, less intrusive approach to immediate newborn care, with intervention only when clearly warranted.22 More research is needed to define best strategies and types of intervention for better outcomes and can only be achieved practically through international collaboration. In Sweden, wide regional variations are observed in mortality and in management. Proactive care did not increase the risk of neurodevelopment impairment at 2.5 years.23 But the definition of pro-active care can be different in different settings.
There is strong evidence that antenatal steroids reduce the risk of BPD and our finding of greater odds of BPD following a complete course of antenatal steroids is likely to be a consequence of survival bias. However, the evidence base for antenatal steroids in women at risk of preterm birth has changed following a trial in low- and middle-income settings that identified harms, including greater risk of neonatal sepsis, itself a risk factor for death and BPD.24 Further, a reappraisal of the Cochrane review evidence has indicated that generalisability across all settings should not be assumed. Though the countries contributing to eNewborn are all high income, they nonetheless are likely to encompass considerable heterogeneity in respect of patient characteristics, no less in care practices.25,26 In addition, many practices have changed since the randomised trials of antenatal steroids were performed, and it is not inconceivable that the balance of benefits to risks may also have altered. Current recommendations for delivery room interventions have changed over time to encourage less invasive techniques.27 Large population data sets such as eNewborn offer potential to re-evaluate treatments over time.
The eNewborn database is in its infancy and has as yet limited coverage. The Vermont Oxford Network started in 1988 and is far larger with a wide range of outputs but also suffers from incomplete population coverage.28 The use of the eNewborn database has thus far been limited to benchmarking. However, a unique aspect is the interactive navigation that has been described elsewhere.4 Data are provided on a collaborative basis and no financial contribution is required by participants. The sustainability of the network is based on external funding from grants, donations and commissions.
The eNewborn platform benefits from flexible data capture and can accommodate direct online recording as well as receive extracts from established databases with quality-assured data, such as the UK National Neonatal Research Database.4,29 Clinicians are rightly and increasingly required to audit care practices against accepted standards, conduct comparative evaluation of outcomes nationally and internationally and undertake quality improvement programmes. The UK Royal College of Paediatrics and Child Health has run a National Neonatal Audit Programme since 2007, and because data for the audit are obtained from the National Neonatal Research Database, clinical teams are not faced with the burdens of added data collection.29 More widespread country-wide participation could drive rigorous pan-European audit especially if overseen by a cross-national authority such as the newly established European Board of Neonatology as part of the European Society of Paediatric Research (https://www.espr.eu/). The introduction of neonatal care standards by the European Foundation for the Care of the Newborn Infant (https://www.efcni.org/) also now provides a potential focus for audit (https://newborn-health-standards.org/). International collaboration can also drive improvements in care by, for example, identifying variation, providing benchmarks and identifying potential areas for improvement such as delivery room resuscitation, as indicated by our findings.
Many newborn interventions have an inadequate evidence base, providing a clear impetus for comparative effectiveness studies. Most neonatal medications continue to be used off-license or off-label, because they have not been evaluated in relevant patient groups. These chronic issues call for sustained emphasis on clinical and translational research. However, we show that it would take a country such as Belgium 9 years and 17 years, respectively, to have a sufficient number of eligible babies to achieve 80% power to detect a statistical significant change in neonatal mortality and BPD following a hypothetical intervention in babies of 23–25 weeks’ GA, in contrast to 1 year for the eNewborn network as a whole. International collaboration offers the only real hope for conducting studies aiming to identify impacts of interventions that are unlikely to have arisen by chance.
In summary, we provide illustrative data describing potential uses of the eNewborn database. Neonatal critical care is a high-cost service that is justified both by the moral imperative to provide quality care for sick newborn infants and because of its life-long impact. The eNewborn platform offers opportunity for high-quality data capture across neonatal services in Europe and the means to drive audit, quality improvement and research.
References
McCormick, M. C., Litt, J. S., Smith, V. C. & Zupancic, J. A. Prematurity: an overview and public health implications. Annu. Rev. Public Health 32, 367–379 (2011).
Costeloe, K. et al. Sharing data to accelerate medicine development and improve neonatal care: data standards and harmonized definitions. J. Pediatr. 203, 437–441 (2018).
Soler, A. et al. EuroNeoStat: a European information system on the outcomes of care for very-low-birth-weight infants. Neonatology 93, 7–9 (2008).
Haumont, D., NguyenBa, C. & Modi, N. eNewborn: the information technology revolution and challenges for neonatal networks. Neonatology 111, 388–397 (2017).
Fenton, T. R. & Kim, J. H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 13, 59 (2013).
Agresti, A. Categorical Data Analysis 2nd edn (John Wiley & Sons, Inc., Hoboken, NJ, 2002).
Allison, P. D. (ed.) Logit Analysis of Contingency Tables. Logistic Regression Using the SAS System: Theory and Application (SAS Institute Inc., Cary, NC, 1999)
Ger, D. & Everitt, B. S. Handbook of Statistical Analyses Using SAS 2nd edn (CRC Press, London, 2001).
Austin, P. C. & Steyerberg, E. W. Interpreting the concordance statistic of a logistic regression model: relation to the variance and odds ratio of a continuous explanatory variable. BMC Med. Res. Methodol. 12, 82 (2012).
Christensen, W. Model Selection Using Information Criteria (Made Easy in SAS®). Paper 2587-2018 (SASGF, Denver, CO, 2018).
Glass, H. C. et al. Outcomes for extremely premature infants. Anesth. Analg. 120, 1337–1351 (2015).
Patel, R. M. Short- and long-term outcomes for extremely preterm infants. Am. J. Perinatol. 33, 318–328 (2016).
Edstedt Bonamy, A. K. et al. Wide variation in severe neonatal morbidity among very preterm infants in European regions. Arch. Dis. Child. Fetal Neonatal Ed. 104, F36 (2019).
American Academy of Pediatrics Committee on Fetus and Newborn & American College of Obstetricians and Gynecologists Committee on Obstetric Practice. The Apgar Score. Pediatrics 136, 819–822 (2015).
Dong, Y. & Peng, C. Y. J. Principled missing data methods for researchers. Springerplus 2, 222 (2013).
Kaempf, J. W., Zupancic, J. A. F., Wang, L. & Grunkemeier, G. L. A risk-adjusted, composite outcomes score and resource utilization metrics for very low-birth-weight infants. JAMA Pediatr. 169, 459–465 (2015).
Horbar, J. D. et al. Variation in performance of neonatal intensive care units in the United States. JAMA Pediatr. 171, e164396 (2017).
Glance, L. G., Dick, A. W., Osler, T. M. & Mukamel, D. Using hierarchical modeling to measure ICU quality. Intensive Care Med. 29, 2223–2229 (2003).
Aelvoet, W. et al. Community-acquired pneumonia (CAP) hospitalization and deaths: is there a role for quality improvement through inter-hospital comparisons. Int. J. Qual. Health Care 28, 22–32 (2016).
Lehtonen, L., Gimeno, A., Parra Llorca, A. & Vento, M. Early neonatal death: a challenge worldwide. Semin. Fetal Neonatal Med. 22, 153–160 (2017).
Hines, D. et al. Scoping review shows wide variation in the definitions of bronchopulmonary dysplasia in preterm infants and calls for a consensus. Acta Paediatr. 106, 366–374 (2017).
Patel, P. N., Banerjee, J. & Godambe, S. V. Resuscitation of extremely preterm infants—controversies and current evidence. World J. Clin. Pediatr. 5, 151–158 (2016).
Serenius, F. et al. Intensity of perinatal care for extremely preterm infants: outcomes at 2.5 years. Pediatrics 135, e1163–e1172 (2015).
Althabe, F. et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet 385, 629–639 (2015).
Reagan, P. B., Salsberry, P. J. & Olsen, R. J. Does the measure of economic disadvantage matter? Exploring the effect of individual and relative deprivation on intrauterine growth restriction. Soc. Sci. Med. 64, 2016–2029 (2007).
Chen, A., Oster, E. & Williams, H. Why is infant mortality higher in the United States than in Europe? Am. Econ. J. Econ. Policy 8, 89–124 (2016).
Foglia, E. E., Jensen, E. A. & Kirpalani, H. Delivery room interventions to prevent bronchopulmonary dysplasia in extremely preterm infants. J. Perinatol. 37, 1171–1179 (2017).
Edwards, E. M. et al. Vermont Oxford Network: a worldwide learning community. Transl. Pediatr. 8, 182–192 (2019).
Battersby, C. et al. The United Kingdom National Neonatal Research Database: a validation study. PLoS ONE 13, e0201815 (2018).
Armitage, P. & Berry, G. Statistical Methods in Medical Research 3rd edn (Blackwell Science Ltd, Oxford, 1994).
Fleiss, J. L. Statistical Methods for Rates and Proportions 2nd edn (John Wiley, New York, 1981).
Acknowledgements
The authors are very grateful to the substantial support of the Baillet-Latour Fund allocated in 2014 to build the eNewborn IT platform The authors wish to thank all participating neonatal units mentioned here for sending their data: Belgian Neonatal Network Antwerp, Ziekenhuis Netwerk Antwerpen (Hilde Van de Broek); Antwerp, Universitair Ziekenhuis Antwerpen (Patrick Van Reempts and Twan Mulder); Antwerp, Sint Augustinus (Christine Vandeputte); Bruges, Algemeen Ziekenhuis Brugge (Luc Cornette); Brussels, Cliniques Universitaires Saint-Luc (Christian Debauche and Olivier Danhaive); Brussels, Chirec (Dominique Grossman); Brussels, Hôpital Erasme (Bart Van Overmeire and Yves Hennequin); Brussels, Hôpital Universitaire des Enfants (Anne Johansson); Brussels, Hôpital Universitaire Saint-Pierre (Marie Tackoen); Brussels, Universitair Ziekenhuis Brussel (Filip Cools); Charleroi, CHU de Charleroi (Eric Cavatorta); Charleroi, Grand Hôpital de Charleroi-Notre Dame (Chantal Lecart); Genk, Ziekenhuis Oost Limburg (Claire Theyskens); Gent, Universitair Ziekenhuis Gent (Koen Smets); La Louvière, CHU de Tivoli (Geneviève Malfilatre); Leuven, Universitair Ziekenhuis Leuven (Gunnar Naulaers); Namur, Centre Hospitalier Régional (Elisabeth Henrion); Liège, CHR Citadelle (Masendu Kalenga and Vincent Rigo); Rocourt, Clinique Saint-Vincent (Pierre Maton). Coordination President College of Physicians: Gunnar Naulaers. Czech Neonatal Network: Ceske Budejovice, Ceske Budejovice Hospital (Markéta Pavlů, Kateřina Matějová); Prague, General University Hospital (Jan Smíšek, Jana Kümmel); Prague, Institute for the Care of Mother and Child (Martin Haša, Peter Korček); Usti nad Labem, Masaryk Hospital (Martin Pánek), Most, Most Hospital (Jana Klesalová), Prague, Thomayer Hospital (Iva Burianová); Zlin, Tomas Bata Regional Hospital (Barbara Tesařová); Brno, University Hospital Brno (Andrea Staníková); Hradec Kralove, University Hospital Hradec Kralove (Zdeněk Kokštejn, Karolína Hanousková); Olomouc, University Hospital Olomouc (Ivana Vránová, Vojtěch Bodnár); Ostrava, University Hospital Ostrava(Hana Burčková); Pilsen, University Hospital Plzen (Martin Matas); Prague, University Hospital in Motol (Jakub Tkaczyk). Network Coordinators: Richard Plavka, Jan Smíšek. Swiss Neonatal Network: Aarau: Cantonal Hospital Aarau, Children’s Clinic, Department of Neonatology (Ph. Meyer, C. Anderegg); Basel: University of Basel Children’s Hospital (UKBB), Department of Neonatology (S. Schulzke); Berne: University Hospital Berne, Department of Neonatology (M. Nelle), Department of Pediatrics (B. Wagner); Chur: Children’s Hospital Chur, Department of Neonatology (W. Bär); Fribourg: Cantonal Hospital Fribourg, Department of Neonatology (G. Kaczala); Geneva: Department of Child and Adolescent, University Hospital (HUG), Neonatology Units (R. E. Pfister); Lausanne: University Hospital (CHUV), Department of Neonatology (J.-F. Tolsa, M. Roth-Kleiner); Lucerne: Children’s Hospital of Lucerne, Neonatal and Pediatric Intensive Care Unit (T. M. Berger); Muensterlingen: Cantonal Hospital Muensterlingen, Department of Pediatrics (B. Erkert, A. Mueller); Neuchatel: Cantonal Hospital Neuchatel, Department of Pediatrics (B. Laubscher); Sion: Service de Pédiatrie de l’Hôpital de Sion (J. Llor); St. Gallen: Cantonal Hospital St. Gallen, Department of Neonatology (A. Malzacher), Children’s Hospital St. Gallen, Neonatal and Paediatric Intensive Care Unit (J. P. Micallef); Winterthur: Cantonal Hospital Winterthur, Department of Neonatology (L. Hegi); Zurich: University Children’s Hospital Zurich, Department of Neonatology (V. Bernet); University Hospital Zurich (USZ), Department of Neonatology (D. Bassler, R. Arlettaz), SwissNeoNet Network Coordinator (M. Adams). Portuguese Neonatal Network: Abrantes, Centro Hospitalar Médio Tejo (João Neves). Almada, Hospital Garcia de Orta (António Gomes; Anselmo Costa; Marta Nunes). Amadora, Hospital Professor Doutor Fernandes Fonseca (Cristina Trindade; Rosalina Barroso; Teresa Aguiar). Angra do Heroísmo, Hospital de Santo Espírito da Ilha Terceira (M. Fátima Nunes). Aveiro, Centro Hospitalar Baixo Vouga (Luís Damas; Paula Rocha; Daniela Pio). Barreiro, Centro Hospitalar Barreiro Montijo (Catarina E. Santo; Joana Extreia). Beja, Unidade Local de Saúde Baixo Alentejo (Fernando Ferreira; Sónia Fernandes). Braga, Hospital de Braga (Almerinda Pereira; Nicole Silva; Carla Sá; Catarina Dias). Bragança, Centro Hospitalar do Nordeste, EPE (Judite Marques). Caldas da Rainha, Centro Hospitalar do Oeste (Jorge Penas). Cascais, Hospital de Cascais (Ana P. Vaz; Manuel Cunha). Castelo Branco, Unidade Local de Saúde de Castelo Branco (Almerinda Silva). Coimbra, Hospital Pediátrico—Centro Hospitalar Universitário de Coimbra (Teresa Dionísio). Coimbra, Maternidade Bissaya Barreto—Centro Hospitalar Universitário de Coimbra (Carlos Lemos; Gabriela Mimoso; Adelaide Taborda; Cristina Resende; Joana Mesquita). Coimbra, Maternidade Doutor Daniel Matos-Centro Hospitalar Universitário de Coimbra (Patrícia Lapa; Rui Castelo). Covilhã, Centro Hospitalar Universitário da Cova da Beira (Ricardo Costa; António Resende; Sandra Mesquita). Évora, Hospital do Espírito Santo de Évora (M. José Mendes; Laura Barroso). Famalicão, Centro Hospitalar Médio Ave (Susana G. Sousa). Faro, Centro Hospitalar Universitário do Algarve (M. José Castro; Ana Mariano; Ermelinda Mendes). Faro, Hospital Particular Algarve (Patrícia Mendes; Luís Gonçalves). Funchal, Hospital Central do Funchal (Henrique Leitão). Guarda, Unidade Local de Saúde da Guarda (Pedro Carvalho). Guimarães, Hospital da Senhora da Oliveira (Alice Freitas; Agostinha Costa; Clara P. Dias). Leiria, Centro Hospitalar de Leiria (Margarida Agostinho; Margarida Pereira). Lisboa, Hospital CUF Descobertas (Mafalda Gonçalves). Lisboa, Hospital D. Estefânia—Centro Hospitalar Lisboa Central (Patrícia Rodrigues; Ema Leal). Lisboa, Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (Margarida Abrantes; Joana Saldanha; Paula Costa). Lisboa, Hospital dos SAMS (Nuno Simões). Lisboa, Hospital Lusíadas Lisboa (Ana Melo Bento; Pedro Silva). Lisboa, Hospital Luz Lisboa (Graça Henriques; Paula Garcia). Lisboa, Hospital S. Francisco Xavier—Centro Hospitalar Lisboa Ocidental (Marta Aguiar; Constança Pinto; Filipa Vieira). Lisboa, Maternidade Dr. Alfredo da Costa—Centro Hospitalar Lisboa Central (Teresa Tomé; Ana Melo; Eduardo Fernandes; Israel Macedo). Loures, Hospital Beatriz Ângelo (Lurdes Oliveira). Matosinhos, Unidade Local de Saúde de Matosinhos (Isabel Martins; Cláudia Ferraz). Penafiel, Centro Hospitalar Tâmega e Sousa (Ana M. Sarmento; Francisco P. Silva). Portimão, Centro Hospitalar Universitário do Algarve (Marta Amado). Porto, Centro Hospitalar Universitário de S. João (Angelina Martins; Paulo Soares). Porto, Centro Materno Infantil do Norte—Centro Hospitalar Universitário do Porto (Alexandra Almeida; Ana Cristina Freitas; Carmen Carvalho; Liliana Pinho; Luísa Neiva). Porto, Hospital CUF Porto (Gabriela Vasconcellos; Maria João Sampaio). Póvoa Varzim, Centro Hospitalar Póvoa Varzim—Vila do Conde (Margarida Pontes). S. Miguel, Hospital do Divino Espírito Santo (M. Fernanda Gomes; Paula Maciel). Santarém, Hospital Distrital de Santarém (José Onofre; José M. Nogueira). Setúbal, Centro Hospitalar de Setúbal (Ana C. Figueiredo). Torres Vedras, Centro Hospitalar do Oeste (Isabel Silva). Viana do Castelo, Unidade Local de Saúde Alto Minho (Armando Laranjeira; Dalila Martins). Vila da Feira, Centro Hospitalar Entre Douro e Vouga (Fátima Fonseca; Teresa Andrade). Vila Franca de Xira, Hospital Vila Franca Xira (Cândida Mendes). Vila Nova de Gaia, Centro Hospitalar Vila Nova Gaia—Espinho (Nise Miranda; Conceição Quintas; Teresa Torres). Vila Real, Centro Hospitalar Trás os Montes e Alto Douro (Eurico Gaspar; Gabriela Pereira; Isabel Soares; Juan Calvino). Viseu, Centro Hospitalar Tondela-Viseu (Isabel Andrade). Coordination: Alexandra Almeida, Rosalina Barroso, Gabriela Mimoso. United Kingdom Neonatal Network: Airedale General Hospital, Airedale NHS Trust (Dr. Matthew Babirecki); Arrowe Park Hospital, Wirral University Teaching Hospital NHS Foundation Trust (Dr. Anand Kamalanathan); Barnet Hospital, Royal Free London NHS Foundation Trust (Dr. Tim Wickham); Barnsley District General Hospital, Barnsley Hospital NHS Foundation Trust (Dr. Kavi Aucharaz); Basildon Hospital, Basildon and Thurrock, University Hospitals NHS Trust (Dr. Aashish Gupta); Basingstoke & North Hampshire Hospital, Hampshire Hospitals NHS Foundation Trust (Dr. Nicola Paul); Bedford Hospital, Bedford Hospital NHS Trust (Dr. Anita Mittal); Birmingham City Hospital, Sandwell and West Birmingham Hospitals NHS Trust (Dr. Penny Broggio); Birmingham Heartlands Hospital, Heart of England NHS Foundation Trust (Dr. Pinki Surana); Bradford Royal Infirmary, Bradford Teaching Hospitals NHS Foundation Trust (Dr. Sunita Seal); Broomfield Hospital, Chelmsford, Mid Essex Hospital Services NHS Trust (Dr. Ahmed Hassan); Calderdale Royal Hospital, Calderdale & Huddersfield NHS Foundation Trust (Dr. Karin Schwarz); Chelsea & Westminster Hospital, Chelsea & Westminster Hospital NHS Foundation Trust (Dr. Shu-Ling Chuang); Chesterfield & North Derbyshire Royal Hospital, Chesterfield Royal Hospital NHS Foundation Trust (Dr. Aiwyne Foo); Countess of Chester Hospital, Countess of Chester Hospital NHS Foundation Trust (Dr. Stephen Brearey); Croydon University Hospital, Croydon Health Services (Dr. John Chang); Cumberland Infirmary, North Cumbria University Hospitals NHS Trust (Dr. Khairy Gad); Darlington Memorial Hospital, County Durham & Darlington NHS Foundation Trust (Dr. Mehdi Garbash); Derriford Hospital, Plymouth Hospitals NHS Trust (Dr. Alex Allwood); Diana Princess of Wales Hospital, Northern Lincolnshire & Goole Hospitals NHS Foundation Trust (Dr. Pauline Adiotomre); Doncaster Royal Infirmary, Doncaster & Bassetlaw Hospitals NHS Foundation Trust (Dr. Nigel Brooke); Dorset County Hospital, Dorset County Hospital NHS Foundation Trust (Dr. Abby Deketelaere); East Surrey Hospital, Surrey and Sussex Healthcare NHS Trust (Dr. K Abdul Khader); Frimley Park Hospital, Frimley Health Foundation Trust (Dr. Sanghavi Rekha); Gloucester Royal Hospital, Gloucestershire Hospitals NHS Foundation Trust (Dr. Simon Pirie); Good Hope Hospital, Heart of England NHS Foundation Trust (Dr. Pinki Surana); Guy’s & St Thomas’ Hospital, Guy’s & St Thomas’ NHS Foundation Trust (Dr. Geraint Lee); Harrogate District Hospital, Harrogate & District NHS Foundation Trust (Dr. Sobia Balal); Hereford County Hospital, Wye Valley NHS Trust (Dr. Cath Seagrave); Hillingdon Hospital, The Hillingdon Hospital NHS Trust (Dr. Tristan Bate); Homerton Hospital, Homerton University Hospital NHS Foundation Trust (Dr. Narendra Aladangady); James Cook University Hospital, South Tees Hospitals NHS Trust (Dr. M Lal); James Paget Hospital, James Paget University Hospitals NHS Foundation Trust (Dr. Ambadkar); Kettering General Hospital, Kettering General Hospital NHS Foundation Trust (Dr. Poornima Pandey); Kings College Hospital, King’s College Hospital NHS Foundation Trust (Dr. Ann Hickey); Kingston Hospital, Kingston Hospital NHS Trust (Dr. Vinay Pai); Lancashire Women and Newborn Centre, East Lancashire Hospitals NHS Trust (Dr. Meera Lama); Leeds Neonatal Service, Leeds Teaching Hospitals NHS Trust (Dr. Lawrence Miall); Leicester General Hospital, University Hospitals of Leicester NHS Trust (Dr. Jonathan Cusack); Leicester Royal Infirmary, University Hospitals of Leicester NHS Trust (Dr. Venkatesh Kairamkonda); Lincoln County Hospital, United Lincolnshire Hospitals NHS Trust (Dr. Kollipara); Lister Hospital, East and North Hertfordshire NHS Trust (Dr. J Kefas); Liverpool Women’s Hospital, Liverpool Women’s NHS Foundation Trust (Dr. Christopher Dewhurst); Luton & Dunstable Hospital, Luton and Dunstable Hospital NHS Foundation Trust (Dr. Jennifer Birch); Manor Hospital, Walsall Hospitals NHS Trust (Dr. Krishnamurthy); Medway Maritime Hospital, Medway NHS Foundation Trust (Dr. Ghada Ramadan); New Cross Hospital, The Royal Wolverhampton Hospitals NHS Trust (Dr. Tilly Pillay); Newham General Hospital, Barts Health (Dr. Imdad Ali); Norfolk & Norwich University Hospital, Norfolk & Norwich University Hospitals NHS Foundation Trust (Dr. Mark Dyke); North Devon District Hospital, North Devon Healthcare NHS Trust (Dr. Michael Selter); North Manchester General Hospital, The Pennine Acute Hospitals NHS Trust (Dr. P Kamath); North Tyneside General Hospital, Northumbria Healthcare NHS Trust (Vivien Spencer); Northampton General Hospital, Northampton General Hospital NHS Trust (Dr. Subodh Gupta); Northwick Park Hospital, London North West Health Care NHS Trust (Dr. Richard Nicholl); Nottingham City Hospital, Nottingham University Hospitals NHS Trust (Dr. Steven Wardle); Nottingham University Hospital (QMC), Nottingham University Hospitals NHS Trust (Dr. Steven Wardle); Ormskirk District General Hospital, Southport and Ormskirk Hospital NHS Trust (Dr. Andreea Bontea); Oxford University Hospitals, John Radcliffe Hospital, Oxford University Hospitals NHS Trust (Dr. Eleri Adams); Peterborough City Hospital, Peterborough and Stamford NHS Foundation Trust (Dr. Katharine McDevitt); Pilgrim Hospital, United Lincolnshire Hospitals NHS Trust (Dr. Ajay Reddy); Pinderfields General Hospital, Mid Yorkshire Hospitals NHS Trust (Dr. David Gibson); Princess Alexandra Hospital, The Princess Alexandra Hospital NHS Trust (Dr. Chinnappa Reddy); Princess Anne Hospital, Southampton University Hospitals NHS Trust (Dr. Freya Pearson); Princess Royal Hospital, Brighton and Sussex University Hospitals NHS Trust (Dr. P Amess); Princess Royal Hospital, Telford, Shrewsbury and Telford Hospital NHS Trust (Dr. Deshpande); Princess Royal University Hospital, King’s College Hospital NHS Foundation Trust (Dr. Elizabeth Sleight); Queen Alexandra Hospital, Portsmouth Hospitals NHS Trust (Dr. Charlotte Groves); Queen Charlotte’s Hospital, Imperial College Healthcare NHS Trust (Dr. Sunit Godambe); Queen Elizabeth Hospital, King’s Lynn, The Queen Elizabeth Hospital King’s Lynn NHS Trust (Dr. Glynis Rewitzky); Queen Elizabeth Hospital, Woolwich, Lewisham and Greenwich NHS Trust (Dr. Olutoyin Banjoko); Queen Elizabeth the Queen Mother Hospital, East Kent Hospitals University NHS Trust (Dr. Abigail Price); Queen’s Hospital, Burton on Trent, Burton Hospitals NHS Foundation Trust (Dr. Dominic Muogbo); Queen’s Hospital, Romford, Barking, Havering & Redbridge University Hospitals NHS Trust (Dr. Khalid Mannan and Dr. Anand Shirsalkar); Rosie Maternity Hospital, Addenbrookes, Cambridge University Hospitals NHS Foundation Trust (Dr. Angela D’Amore); Rotherham District General Hospital, Rotherham NHS Foundation Trust (Dr. Shameel Mattara); Royal Albert Edward Infirmary, Wrightington, Wigan and Leigh NHS Foundation Trust (Dr. Christos Zipitis); Royal Berkshire Hospital, Royal Berkshire NHS Foundation Trust (Dr. Peter De Halpert); Royal Bolton Hospital, Royal Bolton Hospital NHS Foundation Trust (Dr. Paul Settle); Royal Cornwall Hospital, Royal Cornwall Hospitals NHS Trust (Dr. Paul Munyard); Royal Derby Hospital, Derby Teaching Hospitals NHS Foundation Trust (Dr. John McIntyre); Royal Devon & Exeter Hospital, Royal Devon & Exeter NHS Foundation Trust (Dr. David Bartle); Royal Hampshire County Hospital, Hampshire Hospitals NHS Foundation Trust (Dr. Lucinda Winckworth); Royal Lancaster Infirmary, University Hospitals of Morecambe Bay NHS Trust (Dr. Joanne Fedee); Royal Oldham Hospital, The Pennine Acute Hospitals NHS Trust (Dr. Natasha Maddock); Royal Preston Hospital, Lancashire Teaching Hospitals NHS Foundation Trust (Dr. Richa Gupta); Royal Surrey County Hospital, The Royal Surrey County Hospital NHS Trust (Dr. Michael Hardo); Royal Sussex County Hospital, Brighton and Sussex University Hospitals NHS Trust (Dr. P Amess); Royal United Hospital, Royal United Hospital Bath NHS Trust (Dr. Stephen Jones); Royal Victoria Infirmary, Newcastle Upon Tyne Hospitals Foundation Trust (Dr. Alan Fenton); Russells Hall Hospital, Dudley Group of Hospitals NHS Foundation Trust (Dr. Mahadevan); Salisbury District Hospital, Salisbury NHS Foundation Trust (Dr. Nick Brown); Scunthorpe General Hospital, Northern Lincolnshire & Goole Hospitals NHS Foundation Trust (Dr. Pauline Adiotomre); Southend Hospital, Southend University Hospital NHS Foundation Trust (Dr. Vineet Gupta); St George’s Hospital, St George’s Healthcare NHS Trust (Dr. Charlotte Huddy); St Mary’s Hospital, London, Imperial College Healthcare NHS Trust (Dr. Sunit Godambe); St Mary’s Hospital, Manchester, Manchester University NHS Foundation Trust (Dr. Ngozi Edi-Osagie); St Michael’s Hospital, University Hospitals Bristol NHS Foundation Trust (Dr. Pamela Cairns); St Peter’s Hospital, Ashford and St Peter’s Hospitals NHS Trust (Dr. Peter Reynolds); St Richard’s Hospital, Western Sussex Hospitals NHS Trust (Dr. Nick Brennan); Stepping Hill Hospital, Stockport NHS Foundation Trust (Dr. Carrie Heal); Stoke Mandeville Hospital, Buckinghamshire Hospitals NHS Trust (Dr. Sanjay Salgia); Sunderland Royal Hospital, City Hospitals Sunderland NHS Foundation Trust (Dr. Majd Abu-Harb); Tameside General Hospital, Tameside and Glossop Integrated Care NHS Foundation Trust (Dr. Jacqeline Birch); The Jessop Wing, Sheffield, Sheffield Teaching Hospitals NHS Foundation Trust (Dr. Simon Clark); The Royal Free Hospital, Royal Free London NHS Foundation Trust (Dr. Marice Theron); The Royal London Hospital—Constance Green, Barts Health (Dr. Vadivelam Murthy); Torbay Hospital, Torbay and South Devon Healthcare NHS Foundation Trust (Dr. Siba Paul); Tunbridge Wells Hospital, Maidstone & Tunbridge Wells NHS Trust (Dr. Hamudi Kisat); University College Hospital, University College London Hospitals NHS Foundation Trust (Dr. Giles Kendall); University Hospital Coventry, University Hospitals Coventry & Warwickshire NHS Trust (Dr. Kate Blake); University Hospital Lewisham, Lewisham and Greenwich NHS Trust (Dr. Ozioma Obi); University Hospital of North Durham, County Durham and Darlington NHS Foundation Trust (Dr. Mehdi Garbash); Warrington Hospital, Warrington and Halton Hospitals NHS Foundation Trust (Dr. Delyth Webb); Watford General Hospital, West Hertfordshire Hospitals NHS Trust (Dr. Sankara Narayanan); West Middlesex University Hospital, Chelsea and Westminster Hospital NHS Foundation Trust (Dr. Elizabeth Eyre); West Suffolk Hospital, West Suffolk Hospital NHS Trust (Dr. Ian Evans); Wexham Park Hospital, Frimley Health Foundation Trust (Dr. Rekha Sanghavi); Whipps Cross University Hospital, Barts Health (Dr. Caroline Sullivan); Whiston Hospital, St Helens and Knowsley Teaching Hospitals NHS Trust (Dr. Ros Garr); Whittington Hospital, The Whittington Hospital NHS Trust (Dr. Wynne Leith); William Harvey Hospital, East Kent Hospitals University NHS Trust (Dr. Vimal Vasu); Worcestershire Royal Hospital, Worcestershire Acute Hospitals NHS Trust (Dr. Liza Harry); Worthing Hospital, Western Sussex Hospitals NHS Trust (Dr. Katia Vamvakiti); Yeovil District Hospital, Yeovil District Hospital NHS Foundation Trust (Dr. Megan Eaton); York District Hospital, York Teaching Hospitals NHS Foundation Trust (Dr. Sundeep Sandhu); Aberdeen Maternity Hospital, NHS Grampian (Dr. Mike Munro); Cresswell Maternity, Dumfries and Galloway Royal Infirmary, NHS Dunfries and Galloway (Dr. Andrew Eccleston); Cross House Hospital, Kilmarnock, NHS Ayshire and Arran (Dr. S Kinmond); Princess Royal Maternity Hospital, Glasgow, NHS Greater Glasgow and Clyde (Dr. Andrew Powls); Raigmore Hospital, Inverness, NHS Highlands (Dr. P Van Der Heide); Royal Alexandra Hospital, Paisley, NHS Greater Glasgow and Clyde (Dr. Hilary Conetta); Royal Hospital for Children, Glasgow, NHS Greater Glasgow and Clyde (Dr. Morag Campbell); Prince Charles Hospital, Cwm Taf Health Board (Dr. Iyad Al-Muzaffar); Royal Glamorgan Hospital, Cwm Taf Health Board (Dr. Iyad Al-Muzaffar); Royal Gwent Hospital, Aneurin Bevan Health Board (Dr. Siddhartha Sen); Singleton Hospital, Abertawe Bro Morgannwg University Health Board (Dr. Arun Ramachandran); Ysbyty Gwynedd, Betsi Cadwaladr University Health Board (Dr. Mike Cronin); Individual Units: France: Hôpital Clocheville, Tours, Elie SALIBA; Centre Hospitalier Universitaire de Rennes—Hôpital Sud, Patrick Pladys; CHU Arnaud de Villeneuve, Montpelier, Gilles Cambonie; CHU de Limoges, Hôpital de la Mère et de l’Enfant, Antoine Bedu; APHM—Hôpital Nord Marseille, Renaud Vialet; CHU de Lyon-Bron HCL, Olivier Claris; Centre Hospitalier Territorial de Nouvelle Caledonie, Marie-Eve Moulies; Hôpital Necker Enfants-Malades, Paris, Jean-Francois Magny; Centre Hospitalier Intercommunal Créteil, Claude Danan Germany: Universitatsklinikum Essen, Ursula Fielders Hoff-Muser, Anja Stein Spain: Hospital 12 de octobre, Madrid (Carmen Pallas Alonso), Cruces University Hospital Neonatal Unit, Bilbao (Begonia Loureiro). Poland: Medical University of Warsaw, Warsaw (Maria Katarzyna Kornacka). Finland: Kuopio University Hospital, Kuopio (Ulla Sankilampi).
Author information
Authors and Affiliations
Contributions
D.H.: building of the eNewborn network and data collection and drafted the article. N.M.: main partner and contributor in eNewborn network and database, director National Neonatal Research Database NNRD (UK), and participated actively in the writing process. O.D.S.: participated actively in analysis of the results and their relevance. R.A.: effectuated the data management, mapping and contacts with participating units. C.N.: IT responsible and conceptor of eNewborn software platform. M.T.: gave important incentives to neonatal units to participate in eNewborn and contributed in writing the article. K.C.: shared our vision with her experience of databases and supported the development of the network. W.A.: performed all the statistical analyses and discussed the pertinence of the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendices
-
1.
Distribution of the 39,529 patients per national network and the individual units.
N
%
Belgium
3,835
9.7
Czech Republic
2,979
7.5
Portugal
2,965
7.5
Switzerland
2,677
6.8
United Kingdom
25,134
63.6
Individual units
1,945
4.9
-
2.
In order to take sampling fluctuations into account, we adopted a cautious approach wherein we sought to detect a significant difference between true mortality rates π1 and π2, estimated respectively by the ‘actual’ and the ‘future’ mortality rate in the eNewborn database. We tested the null hypothesis that each mortality rate was equal to the pooled mortality rate π. To compute the sample size (Nbr in the table) needed to achieve 80% power to detect a statistically significant change in neonatal mortality and BPD rates in response to a hypothetical intervention, aiming at a 5% change in these rates, we used the formula:30
$${{{N}}\, > \, \left[ \left[\left( {\mathrm{z}}_{2{\upalpha }}{\mathrm{sqrt}}\left(2{\uppi }\left(1 - {\uppi } \right) + {\mathrm{z}}_{2{\upbeta }}{\mathrm{sqrt}}\left( {{\uppi }}1\left( {1 - {\uppi }1} \right) + {\uppi }2\left(1 - {\uppi }2 \right)\right)\right)\right)\right]{/}\left(2{/}{\mathrm{d}}\right)\right]^{2}}$$with Fleiss correction31 of 2/(|π1-π2|),
where N is the number of observations needed, π is the pooled rate of π1, the actual rate, and π2, the decreased rate, d is the difference between actual and future rates, z2α = 1.96 and z2b = 0.8418.
-
3.
Model fit and model selection.
Neonatal mortality | ||||
|---|---|---|---|---|
Model delivery room care | Model 1 min apgar score | Model 5 min apgar score | Evolution apgar score | |
(N = 38.582) | (N = 36.334) | (N = 36.245) | (N = 36.084) | |
C Statistic | 0.840 | 0.847 | 0.851 | 0.856 |
BIC | 2237 | 2501 | 2402 | 3556 |
Loglikelihood | −4993.7 | −4596.3 | −4297.8 | −4939.1 |
Number of parameters | 25 | 25 | 25 | 31 |
BPD | ||||
|---|---|---|---|---|
Model delivery room care | Model 1 min apgar score | Model 5 min apgar score | Evolution apgar score | |
(N = 33.809) | (N = 31.956) | (N = 31.877) | (N = 31.744) | |
C Statistic | 0.848 | 0.849 | 0.851 | 0.848 |
BIC | 3674 | 3769 | 3208 | 5048 |
Loglikelihood | −8664.9 | −9024 | −8508.5 | −9664.8 |
Number of parameters | 27 | 27 | 27 | 33 |
Rights and permissions
About this article
Cite this article
Haumont, D., Modi, N., Saugstad, O.D. et al. Evaluating preterm care across Europe using the eNewborn European Network database. Pediatr Res 88, 484–495 (2020). https://doi.org/10.1038/s41390-020-0769-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0769-x
This article is cited by
-
To intubate or not to intubate, is that the question?
Pediatric Research (2024)
-
Impact of early-onset fetal growth restriction on the neurodevelopmental outcome of very preterm infants at 24 months: a retrospective cohort study
BMC Pediatrics (2023)
-
Developing a resiliency model for survival without major morbidity in preterm infants
Journal of Perinatology (2023)
-
Perinatal Risks of Neonatal and Infant Mortalities in a Sub-provincial Region of China: A Livebirth Population-based Cohort Study
BMC Pregnancy and Childbirth (2022)
-
A birth population-based survey of preterm morbidity and mortality by gestational age
BMC Pregnancy and Childbirth (2021)