Abstract
Background
Painful procedures in early life cause acute pain and can alter pain processing at a spinal level lasting into adulthood. Current methods of analgesia seem unable to prevent both acute and long-term hypersensitivity associated with neonatal pain. The current study aims to prevent acute and long-term hypersensitivity associated with neonatal procedural pain using methadone analgesia in rat pups.
Methods
Sprague–Dawley rat pups received either methadone (1 mg/kg) or saline prior to repetitive needle pricks into the left hind paw from the day of birth (postnatal day (P)0) to P7. Control littermates received a tactile stimulus. Mechanical sensitivity was assessed during the neonatal period (P0–P7), from weaning to adulthood (3–7 weeks) and following surgical re-injury of the same dermatome in adulthood.
Results
Methadone administration completely reversed acute hypersensitivity from P0 to P7. In addition, neonatal methadone analgesia prevented prolonged hypersensitivity after re-injury in adulthood, without affecting sensitivity from weaning to adulthood.
Conclusions
The current study shows that neonatal methadone analgesia can attenuate acute as well as long-term hypersensitivity associated with neonatal procedural pain in a rat model.
Impact
-
Methadone treatment attenuates acute and long-term hypersensitivity associated with neonatal pain in a rat model.
-
Clinical effectiveness studies are urgently warranted to assess acute and long-term analgesic effectivity of methadone.
Similar content being viewed by others
Introduction
Each year, around 15 million infants worldwide are born prematurely and will receive life-saving treatment in the neonatal intensive care unit (NICU).1 During this time, they receive up to 10–14 painful procedures each day.2,3 Despite increased awareness and research into the pain in neonates, infant pain assessment and treatment remains a major clinical challenge. To date, as little as 35% of infants receive analgesia or anaesthesia during these painful procedures.4 Currently, the analgesic treatment in the NICU consists of the three-step ladder of the World Health Organization; starting from non-opioids like acetaminophen to weak opioids (Tramadol) and strong opioids.4 Often used analgesics in the NICU like paracetamol, fentanyl and morphine seem moderately effective at minimizing acute hypersensitivity.5 However, the use of analgesia and especially opioids in early life is still under scrutiny, as they can lead to acute side effects including respiratory distress.5 Importantly, opioids like morphine are not always effective at minimizing pain, as shown recently.6,7 Moreover, the long-term effects of analgesia on neurodevelopment in the presence and absence of pain are still unclear.8,9,10,11,12 Recent evidence shows that perinatal opioids induce neuroapoptosis, anxiety and changes to somatosensation lasting into adulthood.11 In addition, sudden termination of opioid exposure in early life can lead to the debilitating neonatal withdrawal syndrome, leading to symptoms, including respiratory distress, feeding intolerance and seizures.13 Together, this suggests that there is a need for more mechanism-driven analgesia in the neonatal period. Importantly, it is now well documented that painful stimuli in early life result in changes to neurodevelopment lasting into adulthood. Most profoundly, somatosensory processing seems altered, shown as hypersensitivity to re-injury in later life.14,15,16,17,18,19,20 The changes in pain behaviour have been correlated to underlying changes in spinal and supraspinal anatomy and functionality.14,17,21,22 Previously, we have shown that neonatal repetitive procedural pain leads to spinal hyperexcitability, expressed as an increased firing of secondary pain transmission neurons.17 This increase in firing is possibly linked to N-methyl-d-aspartate receptor (NMDA-R)-mediated central sensitization lasting into adulthood.23,24 Mechanism-based analgesia during neonatal pain may prevent these changes. Research shows that the early postnatal spinal cord contains functional µ-opioid receptors (MORs) and NMDA-Rs.25,26 Therefore, methadone becomes an interesting candidate for use as an analgesic. Methadone has a dual functioning of MOR agonism and NMDA-R antagonism,27,28,29 which would make it an ideal drug for analgesia and prevention of central sensitization in the early postnatal period. In the current study, we hypothesized that methadone could reduce acute hypersensitivity during neonatal procedural pain, and could also prevent long-term effects.
Methods
Animals
A total of 64 Sprague–Dawley rat pups were used in this study. Pregnant dams (Charles River) arrived at Maastricht University on gestational day 13 or were mated in-house. Pups were collected after birth and sexed, litters were culled to a maximum of ten pups. Pups of both sexes were randomly assigned to one of three experimental conditions (see “Study design”). After all procedures, pups were returned to the dams as soon as possible to minimize mother–pup separation. At 21 days of age, pups were weaned and housed in same-sex cages of 2–3 animals. Food and water were available ad libitum throughout the entire study. Animals were housed at a reversed 12:12 day–night cycle, in a temperature- (19–24 °C) and humidity- (55 ± 15%) controlled room. All experimental procedures were performed in accordance with the European Directive for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (86/609/EEC) and were approved before commencement of experiments by the Committee for Experiments on Animals, Maastricht, The Netherlands (DEC2017-017).
Study design
To study the effect of methadone analgesia during neonatal repetitive procedural pain, a three-phase approach was used. First, the acute analgesic potency of methadone was assessed by the administration of 1 mg/kg methadone in 0.9% sterile saline (Comfortan 10 mg/ml, Eurovet Animal Health B.V., Bladel, The Netherlands) subcutaneousy (s.c.) before neonatal repetitive needle prick pain. A pilot study (testing 0.4, 1 and 2.5 mg/kg/day in an acute needle prick model experiment; see Supplementary data) showed that this dose of methadone could prevent acute hypersensitivity associated with neonatal needle pricking, without altering behavioural activity or body weight throughout the first postnatal week. The effect of methadone on mechanical sensitivity was assessed from the day of birth (postnatal day (P)0) to P7 and compared to littermates that underwent needle pricks without methadone (Needle prick group, NP) or who underwent tactile stimulation (Tactile control group, TC). To exclude nest-specific effects, the experimental conditions were distributed within each litter (i.e. using littermates as controls). Next, the rat pups were left to recover with the dam until weaning. From weaning to adulthood (P21–P56), mechanical sensitivity was assessed weekly to examine the effect of methadone on global development and baseline sensitivity. In adulthood (8 weeks of age), animals from all three groups underwent an ipsilateral hindpaw incision as a model for acute post-operative pain. Recovery was assessed by testing mechanical sensitivity 1, 3, 5 and 7 days post incision. During all proceedings, caregivers and researchers carrying out behavioural or data analysis were blinded to the treatment condition.
Neonatal repetitive procedural pain model and mechanical sensitivity
As a model for repetitive procedural pain, a repetitive needle prick model was used as previously described.30,31 In short, rat pups (littermates) either received tactile stimulation (cotton swab to the left hind paw, Tactile control (TC), n = 18), noxious needle pricks (NPs, n = 18), or noxious needle pricks with methadone analgesia (1 mg/kg s.c., NP + M, n = 28). A total of four needle pricks (standardized to 2 mm depth with a sterile 25 G needle) or tactile stimuli were administered to the plantar surface of the left hind paw per day in 1-h intervals (8.00, 9.00, 10.00 and 11.00 a.m.) from P0 to P7. Methadone was administered s.c. prior to needle pricks each day; TC and NP animals received s.c. saline to control for injection procedures. Mechanical sensitivity to Von Frey filaments was assessed using the dorsal application of calibrated Von Frey filaments (0.407, 0.692, 1.202, 2.041, 3.63 (from P4 on) and 5.495 (from P6 on) before (baseline, BS) the first needle prick; 1, 3 and 5 h after the last needle prick each day (P0–P7)). Each filament, starting with 0.407, was applied five times to the dorsal surface of the hind paws, until a 5 out of 5 paw withdrawal response was noted. After this, the measurement was completed and no higher filaments were applied. Measures were taken to prevent the filaments from being placed directly onto damaged skin. During the acute phase (P0–P7), Von Frey filaments were applied to the dorsal surface of the paws, while needle prick (or tactile) stimulation was administered to the plantar surface. During procedures, the pups were removed from the cage and placed in a separate box, which was placed under a heating lamp. After procedures, the pups were placed back in the home-cage and dam–nest interaction was observed. Acute maternal care was observed to ensure acceptance of the pups in the home-cage by noting nest building and feeding after the procedures.
Adult re-injury model
At the age of 8 weeks, animals underwent a plantar hindpaw incision as described before.31,32 In short, animals were anaesthetized using isoflurane gas (4–5% induction and 2% maintenance). The skin and fascia on the left hind paw were incised for 1 cm. The underlying plantaris muscle was lifted and incised (1 mm). The skin was closed using two mattress sutures using Ethicon Ethylon sutures with a reverse cutting needle (Ethicon, Somerville, New Jersey). Animals were placed back in their home-cage and monitored during recovery.
Mechanical sensitivity during development and after re-injury in adulthood
After weaning at the age of 3 weeks, mechanical sensitivity was tested weekly until adulthood using Plantar application of Von Frey filaments as described previously.18,21,33 In short, animals were placed individually in Plexiglass chambers on a mesh floor and left to habituate. A range of Von Frey filaments (0.4, 0.6, 1.0, 2.0, 4.0, 6.0, 8.0, 15.0 and 26.0 g) was applied for 5 s onto the mid-plantar surface of both hind paws, starting with 1.0 g. If no response was observed, the filament with a larger force was applied next. If a positive response was observed, the filament with a lower force was applied. This process was repeated until four responses after the initial positive response were recorded. Mechanical sensitivity was tested in the same way before and 1, 3, 5 and 7 days after re-injury at the age of 8 weeks. During the peri-operative phase, the filaments were placed between the callus pads of the paws, to prevent disturbing the surgical wound.
Statistical analysis
Behavioural data were analysed in three separate blocks. The mechanical sensitivity during the neonatal period (P0–P7), the developmental period from 3 to 7 weeks and the peri-operative period in adulthood were all analysed with a repeated-measures analysis of variance with Bonferroni post hoc analysis. Both within- and between-group analysis were run using GraphPad Prism 8.2.1 (GraphPad Software, San Diego, CA, USA). None of the data sets showed a significant difference between male and female rats; therefore, these data were pooled to increase power. All data are plotted as mean ± standard error of the mean (SEM). Asterisks indicate a statistically significant P value of <0.05.
Results
Methadone prevents acute hypersensitivity associated with repetitive needle pricking
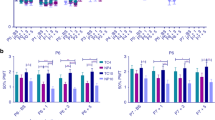
During the first postnatal week, sensitivity was tested daily before (baseline, P0–P7_BS) and 1, 3 and 5 h after (P0–P7_1/3/5) needle pricking. Overall, the paw withdrawal threshold (PWT) increased over time (F (10.40, 384.6) = 10.03; P < 0.0001, Fig. 1). However, an interaction between time and condition was found, showing that not all PWTs increased over time (F (62, 1147) = 4.742; P < 0.0001; Fig. 1). Post hoc analysis revealed that both TC and NP + M showed increased PWTs over time, as expected with the increased weight and skin thickness over the first postnatal week. However, NP PWTs were significantly lower compared to both TC and NP + M from P4 on (overall condition effect: F (2, 37) = 43.92; P < 0.0001; Fig. 1). No statistically significant differences were found between the TC and NP + M groups at any time point.
From P3 on, the NP group shows significantly lower paw withdrawal thresholds compared to both TC and NP + M. TC Tactile control group, NP Needle prick group, NP + M Needle prick + methadone group, PWT paw withdrawal threshold, Pn_BS baseline measurement on postnatal day n; Pn_1/3/5: measurement 1/3/5 h after needle pricks on postnatal day n. Data plotted as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Methadone analgesia during the first postnatal week does not affect mechanical sensitivity after weaning into adulthood
After weaning at 3 weeks of age, mechanical sensitivity was assessed weekly. Overall, PWTs increased over time (F (4, 144) = 67.37, P < 0.0001, Fig. 2). No statistical difference was found between the condition at any time point in mechanical sensitivity (Fig. 2).
Methadone analgesia during the first postnatal week attenuates post-operative hypersensitivity associated with neonatal repetitive procedural pain
At the age of 8 weeks, animals from the TC, NP and NP + M group underwent an ipsilateral hindpaw incision as a model for acute post-operative pain. Overall, the PWT differed over time (within-group analysis; F (2.46, 147.6) = 90.66; P < 0.0001). Although no interaction between time and condition was found (F (8, 240) = 1.384; P = 0.2), the conditions did differ from each other (F (2, 60) = 4.826; P = 0.01). All animals developed acute hypersensitivity seen 1 day after incision (between-group analysis; F (4, 144) = 127.9, P < 0.0001; Fig. 3a). However, a significant condition effect was found (F (2, 36) = 4.390, P < 0.05; Fig. 3a). Post hoc analysis revealed that the PWT of TC animals recovered to baseline levels at post-incision day (PI) 5 (Fig. 3b), while NP animals recovered at PI7 (Fig. 3c). NP + M animals recovered at PI5 (Fig. 3d), suggesting that methadone analgesia during neonatal needle pricking can prevent long-term hypersensitivity to re-injury.
a All groups showed acute hypersensitivity after incision. At day 5, the NP group was significantly more sensitive compared to TC and NP + M. b, c, d show the time course of recovery within each group, showing the change from baseline sensitivity. TC Tactile control group, NP Needle prick group, NP + M Needle prick + methadone group, PWT paw withdrawal threshold, PI post-incisional day. Data are plotted as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Neonatal procedural pain remains a major clinical challenge without adequate and optimized treatment protocols and relative lack of mechanism-based studies on pharmacological and non-pharmacological analgesia. Over the past years, more and more studies have assessed the use of analgesia during early life to prevent the immediate and long-term effects of neonatal pain.31,34 Previously, we have shown that neonatal repetitive procedural pain, modelled as needle pricks in a rat model, leads to acute hypersensitivity and hypersensitivity to re-injury in adulthood.18,30 Here, we show novel evidence that methadone is effective in attenuating both acute and long-term mechanical hypersensitivity in a rat model of neonatal procedural pain.
In our pilot, the tested dosages (0.4, 1 and 2.5 mg/kg/day) were based on literature assessing the analgesic efficacy of methadone in the adult rat.35 These dosages are relatively high as compared to clinically used dosages (30–60 mg/day in adults; max dose of 90–150 mg/day); however, methadone seems to be cleared from the rat much faster than in humans.36 In the current study, the administration of methadone preceding needle pricks in the neonatal period prevented mechanical hypersensitivity observed in the NP group. This effect is most likely due to MOR binding in the spinal dorsal horn, preventing acute neuronal hyperexcitability.37 Calcium imaging in cultured dorsal root ganglia cells showed that neonatal sensory neurons of all fibre types (C, Aβ and Aδ) are sensitive to morphine, whereas only small diameter fibres respond to morphine in adult DRG cells.26,38 In addition, spinal agonism of MOR by synthetic opioid DAMGO inhibits spinal excitability in neonatal rats, confirming that MOR agonism is capable of reducing nociceptive transmission in early neonatal life.26
The current results show methadone administration in a repetitive neonatal needle prick model prevented mechanical hypersensitivity after re-injury in adulthood. Previously, it has been shown that morphine treatment alone during neonatal incisional pain does not prevent long-term hypersensitivity to re-injury after neonatal incision, but does reduce acute hypersensitivity.34 This suggests that merely reducing pain in the early postnatal period is not sufficient at preventing long-term consequences. The fact that methadone administration during neonatal procedural pain results in attenuation of long-term neuronal hyperexcitability might be related to binding to the NMDA-R and thereby reducing NMDA-R-mediated central sensitization lasting into adulthood. The role of the NMDA-R in central sensitization is well documented, and its expression, phosphorylation and subunit composition are highly important in this process.24 Although a subunit switch during postnatal development has been shown to make the NMDA-R more efficient in supraspinal areas,39 it was recently shown that this switch does not occur in the spinal cord dorsal horn (lamina II) in normal, undisturbed early postnatal development (P7–P21).25 It is still unclear whether pathological circumstances, like pain in early life, can alter the subunit expression in the spinal cord, leading to long-term hyperexcitability. Indeed, spinal-wide dynamic range fibres in the dorsal horn show increased excitability following early life pain,17 suggesting central sensitization, either NMDA-R mediated or otherwise. While the prolonged change of NMDA-R-mediated signalling after neonatal pain is yet to be documented, the observed effect of methadone could point to an NMDA-R-mediated mechanism. Perhaps, methadone administration during neonatal repetitive pain can block this process.
Recently, a trial using morphine as analgesia during retinopathy of prematurity screening was stopped prematurely due to the lack of efficacy and severe side effects on respiration in premature infants.6 Therefore, there is a high need for an optimized, mechanism-based treatment approach for procedural pain in the NICU. Clinically, methadone has been used for decades in the NICU in order to treat opioid-related withdrawal symptoms.13,40 Methadone is used rarely as an analgesic in neonates, despite being a promising analgesic with reduced side effects like tolerance.39,41,42,43 Furthermore, antagonism of the NMDA-R makes methadone a unique analgesic which can prevent long-term NMDA-R mediated central sensitization.28,29 The pharmacokinetics of methadone in children, infants and neonates has been studied in detail and seems similar to adults, making the clinical application of methadone in neonates safe and evidence based.44
The current study is not without limitations. First, one of the limitations of this study is the focus on pain behaviour only. Previously, it has been shown that perinatal exposure to methadone can lead to hippocampal neurodegeneration and long-term alterations in memory formation and consolidation.45,46 Additional preclinical research using behavioural measurements could show whether methadone exposure in early life can lead to adverse consequences for developmental milestones and in cognition in later life. However, previous studies have focused on perinatal methadone administration in the absence of pain, for prevention or treatment of neonatal opioid withdrawal syndrome.13,46,47,48 Possibly, when given during painful stimuli, methadone can be neuroprotective as shown before with morphine.49 Second, a downside of the current design is the lack of a non-pharmacological control group in our study design, while treatment of procedural pain with sucrose is used regularly in clinical practice.50 Next, the current design did not allow for full analysis of maternal care (i.e. attention per pup, time spent feeding or grooming), and differences in maternal grooming could have affected the observed outcome. In addition, inflammation and scar formation after repetitive needle pricks could have altered withdrawal responses, and future research should include histological analysis to assess these changes. Finally, as in previous studies, behavioural data of male and female littermates was pooled to increased statistical power,18 while it is clear that the underlying mechanisms may differ between males and females.34,51
In conclusion, this is the first study to show that methadone is effective and attenuates both acute and long-term hypersensitivity caused by repetitive procedural pain in neonates in a rat model. As optimal dosages of methadone in human neonates have been published, clinical studies can shed light on the effectivity of methadone for the prevention of acute and long-term effects of neonatal pain. Although further insights into the underlying mechanism are needed, our behavioural data show that methadone is a promising analgesic to target procedural pain in a vulnerable population.
References
Beck, S. et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull. World Health Organ. 88, 31–38 (2010).
Carbajal, R. et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA 300, 60–70 (2008).
Roofthooft, D. W., Simons, S. H., Anand, K. J., Tibboel, D. & van Dijk, M. Eight years later, are we still hurting newborn infants? Neonatology 105, 218–226 (2014).
Carbajal, R. et al. Sedation and analgesia practices in neonatal intensive care units (EUROPAIN): results from a prospective cohort study. Lancet Respir. Med. 3, 796–812 (2015).
Hall, R. W. Anesthesia and analgesia in the NICU. Clin. Perinatol. 39, 239–254 (2012).
Hartley, C. et al. Analgesic efficacy and safety of morphine in the Procedural Pain in Premature Infants (Poppi) study: randomised placebo-controlled trial. Lancet 392, 2595–2605 (2018).
Monk, V. et al. Oral morphine analgesia for preventing pain during invasive procedures in non-ventilated premature infants in hospital: the Poppi RCT. Southampton (UK): NIHR Journals Library; 2019 Aug. (Efficacy and Mechanism Evaluation, No. 6.9).
Anand, K. J. et al. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet 363, 1673–1682 (2004).
de Graaf, J. et al. Five-year follow-up of effects of neonatal intensive care and morphine infusion during mechanical ventilation on diurnal cortisol rhythm. J. Pediatr. 165, 459–463 e452 (2014).
de Graaf, J. et al. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children’s functioning: five-year follow-up of a randomized controlled trial. Pain 152, 1391–1397 (2011).
Harder, H. J. & Murphy, A. Z. Early life opioid exposure and potential long-term effects. Neurobiol. Stress 10, 100156 (2019).
Valkenburg, A. J. et al. Long-term effects of neonatal morphine infusion on pain sensitivity: follow-up of a randomized controlled trial. J. Pain 16, 926–933 (2015).
Grossman, M. & Berkwitt, A. Neonatal abstinence syndrome. Semin. Perinatol. 43, 173–186 (2019).
Beggs, S., Currie, G., Salter, M. W., Fitzgerald, M. & Walker, S. M. Priming of adult pain responses by neonatal pain experience: maintenance by central neuroimmune activity. Brain 135, 404–417 (2012).
Walker, S. M. et al. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain 141, 79–87 (2009).
den Hoogen, N. J. V., Patijn, J., Tibboel, D. & Joosten, E. A. Neonatal plasticity of the nociceptive system: mechanisms, effects, and treatment of repetitive painful procedures during NICU admittance. Curr. Pharm. Des. 23, 5902–5910 (2017).
van den Hoogen, N. J. et al. Repeated touch and needle-prick stimulation in the neonatal period increases the baseline mechanical sensitivity and postinjury hypersensitivity of adult spinal sensory neurons. Pain 159, 1166–1175 (2018).
van den Hoogen, N. J., Patijn, J., Tibboel, D. & Joosten, E. A. Repetitive noxious stimuli during early development affect acute and long-term mechanical sensitivity in rats. Pediatr. Res. 87, 26–31 (2019).
Ren, K., Novikova, S. I., He, F., Dubner, R. & Lidow, M. S. Neonatal local noxious insult affects gene expression in the spinal dorsal horn of adult rats. Mol. Pain 1, 27 (2005).
Walker, S. M. Long-term effects of neonatal pain. Semin. Fetal Neonatal Med. 24, 101005 (2019).
van den Hoogen, N. J., van Reij, R. R., Patijn, J., Tibboel, D. & Joosten, E. A. J. Adult spinal opioid receptor mu1 expression after incision is altered by early life repetitive tactile and noxious procedures in rats. Dev. Neurobiol. 78, 417–426 (2018).
Laprairie, J. L. & Murphy, A. Z. Neonatal injury alters adult pain sensitivity by increasing opioid tone in the periaqueductal gray. Front. Behav. Neurosci. 3, 31 (2009).
Schwaller, F. & Fitzgerald, M. The consequences of pain in early life: injury-induced plasticity in developing pain pathways. Eur. J. Neurosci. 39, 344–352 (2014).
Woolf, C. J. Central sensitization: uncovering the relation between pain and plasticity. Anesthesiology 106, 864–867 (2007).
Mahmoud, H., Martin, N. & Hildebrand, M. E. Conserved contributions of NMDA receptor subtypes to synaptic responses in lamina II spinal neurons across early postnatal development. Mol. Brain 13, 31 (2020).
Kwok, C. H., Devonshire, I. M., Bennett, A. J. & Hathway, G. J. Postnatal maturation of endogenous opioid systems within the periaqueductal grey and spinal dorsal horn of the rat. Pain 155, 168–178 (2014).
Ebert, B., Thorkildsen, C., Andersen, S., Christrup, L. L. & Hjeds, H. Opioid analgesics as noncompetitive N-methyl-D-aspartate (NMDA) antagonists. Biochem. Pharmacol. 56, 553–559 (1998).
Sotgiu, M. L., Valente, M., Storchi, R., Caramenti, G. & Biella, G. E. Cooperative N-methyl-D-aspartate (NMDA) receptor antagonism and mu-opioid receptor agonism mediate the methadone inhibition of the spinal neuron pain-related hyperactivity in a rat model of neuropathic pain. Pharm. Res. 60, 284–290 (2009).
Gorman, A. L., Elliott, K. J. & Inturrisi, C. E. The d- and l-isomers of methadone bind to the non-competitive site on the N-methyl-D-aspartate (NMDA) receptor in rat forebrain and spinal cord. Neurosci. Lett. 223, 5–8 (1997).
Knaepen, L. et al. Neonatal repetitive needle pricking: plasticity of the spinal nociceptive circuit and extended postoperative pain in later life. Dev. Neurobiol. 73, 85–97 (2013).
van den Hoogen, N. J. et al. Neonatal paracetamol treatment reduces long-term nociceptive behaviour after neonatal procedural pain in rats. Eur. J. Pain 20, 1309–1318 (2016).
Brennan, T. J., Vandermeulen, E. P. & Gebhart, G. F. Characterization of a rat model of incisional pain. Pain 64, 493–501 (1996).
Chaplan, S. R., Bach, F. W., Pogrel, J. W., Chung, J. M. & Yaksh, T. L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 (1994).
Moriarty, O., Harrington, L., Beggs, S. & Walker, S. M. Opioid analgesia and the somatosensory memory of neonatal surgical injury in the adult rat. Br. J. Anaesth. 121, 314–324 (2018).
Holtman, J. R. Jr. & Wala, E. P. Characterization of the antinociceptive and pronociceptive effects of methadone in rats. Anesthesiology 106, 563–571 (2007).
Ling, G. S., Umans, J. G. & Inturrisi, C. E. Methadone: radioimmunoassay and pharmacokinetics in the rat. J. Pharm. Exp. Ther. 217, 147–151 (1981).
Smith, T. H., Grider, J. R., Dewey, W. L. & Akbarali, H. I. Morphine decreases enteric neuron excitability via inhibition of sodium channels. PLoS ONE 7, e45251 (2012).
Nandi, R. et al. The functional expression of mu opioid receptors on sensory neurons is developmentally regulated; morphine analgesia is less selective in the neonate. Pain 111, 38–50 (2004).
Liu, X. B., Murray, K. D. & Jones, E. G. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J. Neurosci. 24, 8885–8895 (2004).
Turner, S. D. et al. Neonatal opioid withdrawal and antenatal opioid prescribing. CMAJ Open 3, E55–E61 (2015).
Barnett, A. M. et al. The effect of intraoperative methadone during pediatric cardiac surgery on postoperative opioid requirements. Paediatr Anaesth. 30, 773–779 (2020).
Chana, S. K. & Anand, K. J. Can we use methadone for analgesia in neonates? Arch. Dis. Child Fetal Neonatal Ed. 85, F79–F81 (2001).
Anand, K. J. Pharmacological approaches to the management of pain in the neonatal intensive care unit. J. Perinatol. 27, S4–S11 (2007).
Ward, R. M. et al. The pharmacokinetics of methadone and its metabolites in neonates, infants, and children. Paediatr. Anaesth. 24, 591–601 (2014).
Schrott, L. M., Franklin, L. & Serrano, P. A. Prenatal opiate exposure impairs radial arm maze performance and reduces levels of BDNF precursor following training. Brain Res. 1198, 132–140 (2008).
Chen, H. H. et al. Buprenorphine, methadone, and morphine treatment during pregnancy: behavioral effects on the offspring in rats. Neuropsychiatr. Dis. Treat. 11, 609–618 (2015).
Fjelldal, M. F. et al. Opioid receptor-mediated changes in the NMDA receptor in developing rat and chicken. Int. J. Dev. Neurosci. 78, 19–27 (2019).
Vestal-Laborde, A. A., Eschenroeder, A. C., Bigbee, J. W., Robinson, S. E. & Sato-Bigbee, C. The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination. Dev. Neurosci. 36, 409–421 (2014).
Duhrsen, L. et al. Effects of repetitive exposure to pain and morphine treatment on the neonatal rat brain. Neonatology 103, 35–43 (2013).
Stevens, B., Yamada, J., Ohlsson, A., Haliburton, S. & Shorkey, A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst. Rev. 7, CD001069 (2016).
Moriarty, O. et al. Priming of adult incision response by early-life injury: neonatal microglial inhibition has persistent but sexually dimorphic effects in adult rats. J. Neurosci. 39, 3081–3093 (2019).
Author information
Authors and Affiliations
Contributions
N.v.d.H., J.P., D.T. and E.A.J. contributed to conception and design of the study. N.v.d.H. and T.J.d.G. collected the data. N.v.d.H. analysed the data and drafted the manuscript. N.v.d.H., T.J.d.G., J.P., D.T. and E.A.J. read and revised the manuscript, and provided final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
van den Hoogen, N.J., de Geus, T.J., Patijn, J. et al. Methadone effectively attenuates acute and long-term consequences of neonatal repetitive procedural pain in a rat model. Pediatr Res 89, 1681–1686 (2021). https://doi.org/10.1038/s41390-020-01353-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01353-x