Abstract
Background
To study the risk factors and outcomes of severe acute kidney injury (AKI) in neonates with necrotizing enterocolitis.
Methods
Retrospective chart review of 202 neonates with necrotizing enterocolitis (NEC) (Bell stage >IIa) from 2013 to 2018. AKI was defined as per-modified neonatal Kidney Disease: Improving Global Outcomes criteria. Demographic, clinical, and outcome data were compared between neonates without severe AKI (stage 0 and 1 AKI) and those with severe AKI (stage 2 and 3 AKI).
Results
Severe AKI occurred in 66/202 (32.6%) of neonates after NEC diagnosis and after 61/104 (58.7%) of surgical NEC diagnoses. On adjusted model, surgical NEC [adjusted odds ratio (aOR) = 30.6; 95% confidence interval (CI) = 8.9, 130.6], outborn [aOR = 3.9; 95% CI = 1.54, 11.0], exposure to antenatal steroids [aOR = 3.0; 95% CI = 1.1, 8.9], and positive blood culture sepsis [aOR = 3.5; 95% CI = 1.3, 10.0] had increased odds for severe AKI. Those with severe AKI required longer hospitalization [124 days (interquartile range (IQR) 88–187) vs. 82 days (IQR 42–126), p < 0.001].
Conclusions
Severe AKI is common in neonates with NEC who require surgical intervention, are outborn, have positive blood culture sepsis, and receive antenatal steroids. Severe AKI is associated with a significantly longer length of hospitalization.
Impact
-
Neonates with NEC, who are transferred from outside hospitals, require surgical NEC management, and/or have a positive blood culture at NEC onset are at the highest odds for severe (stages 2 and 3) AKI.
-
Assessment of urine output is important for patients with NEC. Without it, 11% of those with severe AKI would have been misdiagnosed using serum creatinine alone.
-
Kidney-protective strategies in the pre-, peri-, and postoperative period may improve the morbidity and mortality associated with severe AKI in neonates with NEC.
Similar content being viewed by others
Introduction
Necrotizing enterocolitis (NEC) is the most common acute gastrointestinal illness during the neonatal period, affecting ~5–10% of premature neonates with a birth weight ≤1500 g.1,2 NEC remains a leading cause of morbidity and mortality among premature neonates and leads to increased costs of care and resource utilization.3,4,5,6,7,8,9 NEC is associated with severe systemic inflammatory response and a septic shock-like clinical picture that can contribute to multiorgan dysfunction due to capillary leak syndrome, intravascular volume depletion, and hypotension. These hemodynamic changes and the use of nephrotoxic medications (such as vancomycin and aminoglycosides) can lead to acute kidney injury (AKI), especially in underdeveloped preterm kidneys. One of the most commonly used animal models of AKI (the cecal ligation and puncture model) has many features similar to NEC with a clinical picture of bacterial peritonitis.10 In addition, NEC mouse model studies by Garg et al.11 have demonstrated that NEC is associated with widespread kidney inflammation and AKI.
AKI during the neonatal period affects ~30% of sick neonates and is associated with poor clinical outcomes in premature and term neonates.12,13 AKI following NEC has been previously reported.14,15,16,17 However, prior studies examining associations between neonatal AKI and NEC are limited due to sample size, and the lack of urine output (UOP) criteria in the diagnosis of AKI; a limitation that increases the likelihood of AKI misclassification in some affected neonates.11,18,19,20
In this single-center, retrospective observational cohort study, we sought to determine the demographics, clinical parameters, and interventions that were independently associated with severe AKI in premature neonates with NEC. We compare changes in weight, sodium, and serum creatinine (SCr) after NEC in those with and without severe AKI. Our primary hypothesis is that severe AKI is associated with prolonged length of hospital stay and mortality.
Methods
Population and study design
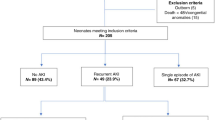
The study was conducted at the neonatal intensive care unit (NICU) at the University of Mississippi Medical Center (UMMC). The NICU is a Level IV unit with ~1000 admissions annually and referrals from throughout the state. This retrospective cohort study was approved by the UMMC Institutional Review Board evaluating neonates, with a waiver of informed parental consent. All neonates admitted between January 2013 and December 31, 2018, with a diagnosis of NEC (Bell stage II and above) were included in the study.21 Neonates with a diagnosis of spontaneous intestinal perforation, congenital heart disease, intestinal atresia, kidney anomalies, and missing clinical data were excluded from the analysis.
Demographic variables
We collected demographic data, which includes gestational age (GA), birth weight (BW), sex, appropriate for gestational age status (AGA), race, outborn status, and mode of delivery. We recorded Apgar scores ≤6 at 5 min as our institution utilizes this cut-off to assess hypoxia and hemodynamic instability. We also collected maternal variables, which included maternal pregnancy-induced hypertension (PIH), chorioamnionitis, and antenatal steroids.
NEC variables
We recorded information on the age (in days) at the time of NEC diagnosis. The diagnosis of NEC was made by abdominal X-ray by board-certified pediatric radiologists based on radiological NEC findings, including pneumatosis, portal venous gas, and pneumoperitoneum. The frequency of surgical NEC (Bell stage III) was also collected.21 Neonates who died within 48 h after NEC onset were classified as having fulminant NEC per literature and our center guidelines.22 We also classified infants as having fulminant NEC if massive bowel necrosis was found during laparotomy or autopsy. Our surgical team and their approach to the surgical management NEC remained unchanged throughout the course of the study period. Patients with NEC were managed surgically if they had clinical worsening due to intestinal perforation, hypotension, persistent electrolyte imbalances (e.g., hyperkalemia, metabolic acidosis), increasing ventilatory support, or intractable anemia and thrombocytopenia, despite repeated red blood cell and platelet transfusions. We also collected the frequency of cholestasis (serum bilirubin >2 mg/dL) at the time of NEC diagnosis.
Hemodynamic variables
Additional clinical information included mechanical ventilation exposure, patent ductus arteriosus (PDA), frequency of PDA surgical ligation, inotropes (dopamine) use 24 h after NEC onset, serum sodium <135 meq/L at 24 h after NEC onset, and indomethacin/ibuprofen therapy (before NEC).
Sepsis variables
Sepsis-related variables included blood culture-proven sepsis at the time of NEC onset and type of antibiotics (vancomycin, gentamicin, piperacillin/tazobactam, metronidazole, amikacin, and fluconazole) used for 2 weeks following NEC diagnosis. The choices of antibiotics at our center for infants with NEC are vancomycin, amikacin, and metronidazole. In a few neonates who experienced persistent thrombocytopenia, suspicion of fungal infection led to the addition of a prophylactic fluconazole regimen. In addition, several neonates referred to our center were receiving gentamicin. We also analyzed the total duration of antibiotics in days for all antibiotics previously mentioned.
Kidney function data
We examined all SCr measurements, daily UOP, serum sodium levels, and weight the day before NEC diagnosis, at NEC onset, and at 24, 48, 72, and 96 h after NEC diagnosis. We also examined these variables at 7 and 14 days after NEC diagnosis. The incidence of AKI within the 14 days after NEC onset was determined using the modified neonatal staging criteria as previously described in the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for AKI.12,23,24,25,26 Baseline SCr was defined by the lowest SCr documented in the patient’s clinical record before NEC onset. Stage 1 AKI was defined as a rise in SCr by 0.3 mg/dL or a rise by 1.5–1.9 times above baseline and/or UOP <1 mL/kg/h over the last 24 h. Stage 2 AKI was defined as an increase in SCr 2–2.9 times above baseline and/or UOP <0.5 mL/kg/h. Stage 3 AKI was defined as an increase in SCr three times above baseline or SCr >2.5 mg/dL and/or and UOP <0.3 mL/kg/h.26 The maximum AKI stage was defined as the highest SCr or UOP within 14 days after the NEC onset. The UMMC hospital laboratories measure SCr using the isotope dilution mass spectrometry method. The UOP estimated using weighed diapers recorded by bedside nurses in the electronic medical record. We stratified neonates as without severe (no AKI or stage 1 AKI) and with severe (stage 2 and 3 AKI) as has been done in previous neonatal and pediatric studies.12,27
Outcome data
The primary outcome was the development of severe AKI (stage 2 and 3 AKI). To assess the impact of severe AKI on outcomes, length of stay and hospital mortality were measured. The length of stay was defined as the total duration of hospitalization from the day of admission until discharge or death. Mortality was defined as death due to any cause before hospital discharge.
Statistical methods
For normally distributed continuous variables, we summarized the data as mean and standard deviation (±SD), and comparisons between those without severe AKI and with severe AKI were performed using Student’s t test and analysis of variance. For continuous data exhibiting skewed distribution, median with interquartile range (IQR) [1st quartile; 3rd quartile] are presented and differences in the data were tested using Mann–Whitney U test or Kruskal–Wallis test. Categorical data were summarized as counts with relative frequencies as percentages and differences in the groups were analyzed using the χ2 test or Fisher’s exact test. Risk factors of severe AKI were analyzed with univariate and multivariate logistic regression. No variable selection strategy was adopted. Variables were chosen in the model due to their clinical importance for examining severe AKI infants. First, we performed a univariate logistic regression analysis to examine the crude association between each of the risk factors and the development of severe AKI. Following this, an adjusted model was developed using the variables in the univariate logistic regression in the multivariate logistic regression analysis. To avoid multicollinearity, BW was not included in the model since it exhibited a moderately significant positive correlation (Pearson product–moment correlation coefficient, r = 0.62, p value < 0.001) with GA. The variables within the logistic regression models were expressed as odds ratios with 95% confidence intervals (CIs). In a secondary analysis, we compared mortality among neonates without AKI to those with any stage AKI. A p value < 0.05 was considered statistically significant for all the analyses. All the statistical analyses were performed in R statistical software (version 3.6.3; The R Foundation for Statistical Computing).
Results
Demographic variables
Of the 230 neonates, we screened with the diagnosis of possible NEC, 202 met the inclusion and exclusion criteria, while 28 were excluded, as they had an inconclusive diagnosis of NEC. The cohort had a mean GA of 28.3 ± 4.4 weeks, a mean BW of 1191 ± 767 g, was predominantly male (59.4%), African American (72.6%), and outborn (58.7%). Additional demographic characteristics are summarized in Table 1.
NEC variables
The mean age of NEC onset was 21.4 ± 21.4 days. Radiological findings on abdominal x-ray showed pneumatosis in 88/200 (44%), pneumoperitoneum 56/198 (28.3%), and portal venous gas in 9/198 (4.5%) cases. One hundred and four (51.5%) of all neonates developed surgical NEC, and 74/104 (71.2%) had ostomy creation after surgical intervention. Cholestasis was seen in 66/119 (55.5%) at NEC onset.
Hemodynamic variables
At the time of NEC onset, 144/202 (73.5%) required assisted ventilation, and 113/198 (57.4%) required inotropic support with dopamine at 24 h after NEC onset. Co-morbid conditions and interventions of the cohort include 98/200 (49%) with patent ductus arteriosus, in whom 23/175 (13.1%) required medical treatment with cyclooxygenase inhibitors (indomethacin/ibuprofen) before NEC onset.
Sepsis variables
Among the neonates of this cohort, 59/201 (29.4%) had blood culture-positive sepsis at the time of the NEC onset, and required antibiotics for a mean time of 8.1 ± 4.4 days following diagnosis.
Acute kidney injury
Of the 202 neonates, 94/202 (46.5%) had AKI, 108/202 (53.5%) had no AKI, 28/202 (13.9%) had stage 1, 36/202 (17.8%) had stage 2, and 30/202 (14.9%) had stage 3 AKI by highest SCr or UOP staging criteria. Combining stages 2 and 3 (severe AKI) occurred in 66/202 (32.7%), while 136/202 (67.3%) had stage 0 and 1 AKI. Using only a change in SCr to define AKI, 71/202 (35.1%) had any AKI, 34 (16.8%) stage 1, 18 (8.9%) stage 2, and 19 (9.4%) with stage 3. Using the KDIGO UOP criteria alone, 50/202 (24.7%) had any AKI, 4 (1.9%) had stage 1, 31 (15.3%) had stage 2, and 15 (7.4%) had stage 3. Forty-four neonates (62%) met the KDIGO diagnostic criteria for AKI by SCr, but did not meet the criteria for AKI by UOP, while 23 neonates (11.4%) met the criteria for AKI by UOP alone despite a stable and/or normal SCr (Table 2).
Table 1 also compares baseline demographic characteristics, as well as NEC features, hemodynamic factors, and infectious factors between participants defined with (stages 2 and 3) and without (stages 0 and 1) severe AKI by SCr and/or UOP criteria. On the bivariate analysis, compared to those with without severe AKI, neonates with severe AKI had significantly lower GA (26.9 ± 4.2 vs. 29.0 ± 4.4 weeks, p = 0.002), lower BW (927 ± 531 vs. 1320 ± 830 g, p < 0.001), were more likely to be outborn [73% vs. 51.9%, p = 0.008], had significantly greater exposure to maternal PIH [27.9% vs. 12.9%, p = 0.02], and antenatal steroid exposure [74.2% vs. 55%, p = 0.02]. Neonates with severe AKI were also more likely to have had an x-ray demonstrating pneumoperitoneum [42.2% vs. 21.6%, p = 0.005] to have required a surgical intervention [92.4% vs. 31.6%, p < 0.001], to have received an ostomy [85% vs. 33.8%, p < 0.001), and experienced cholestasis [73.7% vs. 38.7%, p < 0.001] compared to neonates without severe AKI. Any assisted ventilation [(97% vs. 86.8%, p < 0.001], dopamine use at 24 h after NEC onset [78.5% vs. 47%, p < 0.001], serum sodium <135 meq/L at 24 h after NEC onset [53.8% vs. 34.7%, p = 0.02], blood culture-positive sepsis [42.4% vs. 23%, p = 0.007], and use of metronidazole [50.8% vs. 31.3%, p = 0.01] were significantly more common among neonates with severe AKI. Gentamicin was the only antibiotic that was significantly more commonly used in infants without severe AKI [48.9% vs. 24.2%, p = 0.001].
Table 3 shows the unadjusted and adjusted odds of developing severe AKI. In the unadjusted risk factor analysis for severe AKI, lower BW, lower GA, surgical NEC, positive blood culture at NEC diagnosis, outborn status, pneumoperitoneum, exposure to PIH, and exposure to antenatal steroids were associated with a higher odds of severe AKI in neonates. However, gentamicin use was associated with lower odds of developing severe AKI. In the adjusted risk factor analysis, after controlling for multiple confounders, exposure to antenatal steroids [adjusted odds ratio (aOR) 3.05, 95% CI 1.1–8.9, p = 0.03], outborn status (aOR 3.9, 95% CI 1.5–11.0, p = 0.006), surgical NEC (aOR 30.7, 95% CI 9.0–130.7, p < 0.001), and positive blood culture at the time of NEC diagnosis (aOR 3.5, 95% CI 1.3–10.0, p = 0.02) were associated with increased odds of severe AKI. Gentamicin exposure (aOR 0.31, 95% CI 0.1–0.9, p = 0.03) was associated with decreased odds of developing severe AKI in this cohort.
Trends of SCr, UOP, serum sodium, and anthropometric values for the cohort categorized by AKI status are demonstrated in Fig. 1 (Supplementary Table 1). The baseline SCr did not differ between groups. As expected, at NEC diagnosis and at 24 h after NEC diagnosis, those with severe AKI had higher SCr values compared to those without severe AKI (0.90 mg/dL ± 0.42 vs. 0.61 mg/dL ± 0.3, p < 0.0001 at NEC onset and 0.93 mgdL ± 0.5 vs. 0.60 mgdL ± 0.3, p < 0.0001 24 h after NEC diagnosis). Among those with severe AKI, the mean SCr levels remained significantly increased until 96 h after NEC onset (p < 0.001) and then began to approach a nadir between after 96 h to 2 weeks following NEC onset. The UOP decreased on the day of NEC onset to a more significant degree in those with severe AKI vs. those without severe AKI [decreased from 3.99 mL/kg/h (±1.4) to 2.02 mL/kg/h (±1.4) vs. decreased from 3.87 mL/kg/h (±1.3) to 3.35 mL/kg/h (±1.2), p < 0.001]. In those with severe AKI, UOP remained decreased for 24 h after NEC onset compared to those without severe AKI neonates (p < 0.001). Importantly, as opposed to SCr, which took days to improve, the UOP began to improve 24 h after NEC onset. Compared to those without severe AKI, those with severe AKI had lower median serum sodium at 24 h after NEC [134 meq/L (IQR 129, 140) vs. 136 meq/L (IQR 132, 140), p = 0.005]. Serum sodium levels improved to within normal reference ranges and were no longer significantly different within 48 h after NEC onset.
Trend of Serum creatinine (a), Urine Output (b), Serum Sodium (c) and Anthropometric Parameters (d) at Different Time Points Before and After NEC Onset among neonates with and without severe AKI in the neonatal intensive care unit, 2013–2018. The astrerix (*) on X-axis represents p < 0.05 at that time point after NEC onset. Zero (0) day on X-axis represents the day of NEC onset.
To assess the weight change in neonates with NEC and AKI, we evaluated mean weight and weight change from before NEC onset to >72 h and >7 days after NEC onset (Table 4). The mean weight was significantly lower in neonates with severe AKI group compared to neonates without severe AKI at all the time assessment points (p = 0.001). The percentage weight change at 72 h [1.19 (0; 4.3)% vs. 0 (0; 0.5)%, p = 0.017] was statistically significantly greater in infants with without severe AKI group. However, the percentage weight change at 7 days [5.0 (0; 13.8)% vs. 1.98 (0; 11.4)%, p = 0.94)] after NEC onset was not significantly different between the two groups (also see Supplementary Table 1).
Length of hospitalization
The median length of hospitalization was 95 days (IQR 48; 151) in our cohort. Compared to those without severe AKI, those with severe AKI had a significantly longer length of hospitalization [81.5 days (42.2, 126) vs. 124 days (IQR 87.5, 187), p < 0.001].
Mortality
Fifty-one neonates (25.2%) died in our study population following the diagnosis of NEC. Compared to those without severe AKI, those with severe AKI experienced a slightly higher frequency of death (24.3% vs. 27.3%), but this difference did not reach statistical significance (p = 0.77). In a secondary analysis, we compared mortality among neonates without AKI to those with any stage AKI. Those with any stage AKI experienced a higher frequency of death [29/94 (30.9%) vs. 22/108 (20.4%)], but this difference also did not reach statistical significance (p = 0.12).
Discussion
Our study is the first study to evaluate AKI in neonates with NEC using a contemporary AKI definition that incorporates both SCr and UOP data. We found that severe AKI occurs in ~1 of every 3 all neonates with NEC (medical and surgical). Among neonates who develop surgical NEC, as many as 60% experienced severe AKI. The incidence of severe AKI in our study (32%) was greater than the incidence of stage 2–3 AKI from the Assessment of Worldwide Acute Kidney injury Epidemiology in Neonates (AWAKEN) study (16%), the largest study of neonatal AKI. However, the number of participants in the AWAKEN study with NEC was small (n = 17 of 2022). Importantly, we show that 11% of the cohort with AKI would have been misclassified as not having AKI based on SCr alone. This is supported by similar findings from the AWAKEN study, which demonstrated 14% of infants diagnosed with AKI Assessment of Worldwide Acute Kidney Injury, Renal Angina and Epidemiology in Critically Ill Children (AWARE) study, which demonstrated that 10.7% of children would have been missed without the assessment of UOP.12,28 Alternatively, 62% of the cohort could have been misclassified as not having AKI based on UOP alone, emphasizing the importance of regular assessment of UOP, and SCr among neonates with NEC.
The trend of SCr and UOP for neonates in our study demonstrates that the rise in SCr occurs within 24 h after NEC onset in most neonates and the SCr elevation persists upwards of 96 h after NEC diagnosis in those with severe AKI. Our data support findings in mice models, demonstrating that there are kidney physiologic changes following a diagnosis of NEC. Given that surgical NEC is a risk factor for severe AKI, these kidney physiologic changes are likely most pronounced in the postoperative period. This highlights the importance of consideration and implementation of kidney-protective strategies following a diagnosis of NEC and particularly among neonates with surgical NEC.
Other studies have reported risk factors associated with AKI and clinical outcomes in neonates with NEC.18,19,20 Bakoum et al. studied 77 neonates with Bell’s stage II/III NEC and reported AKI in 42.9% of neonates (stage 1: 18.2%; stage 2: 13%; stage 3: 11.7%). Similar to our study, worsening severity of NEC (surgical NEC), was associated with AKI. Although, even after controlling for potential confounders, those neonates in our study with exposure to antenatal steroids, outborn status, and blood culture-positive sepsis at the time of NEC, as well as surgical NEC, had a significantly greater odds of experiencing severe AKI. This likely indicates the neonates with severe AKI, in this study, may have been the more critically ill and hemodynamically unstable neonates, rather than these factors being directly nephrotoxic.
The risk of nephrotoxicity related to neonatal gentamicin exposure has been previously well described.29 However, in this study, gentamicin exposure was associated with decreased odds of severe AKI. We speculate that this is likely due to less cumulative exposure to gentamicin in our center rather than a kidney-protective effect from gentamicin. Neonates with severe AKI group were also more likely to have late-onset NEC disease and received amikacin at a greater frequency than gentamicin, for example.
We also found that neonates with severe AKI experienced longer NICU hospitalizations with a median difference of 40 days between groups. Bakoum et al. reported that the length of stay was not significantly longer among the neonates with AKI compared to the neonates without AKI [10 days (50.5, 128) vs. 67 days (42, 94), respectively, p = 0.09].19 We suspect that our study demonstrated differences in the length of stay because of the larger number of neonates with severe AKI in our cohort, which likely contributed to a longer length of hospitalization beyond what is generally required of neonates experiencing NEC alone. Multiple studies have shown that severe AKI is associated with prolonged hospitalizations in other critically ill pediatric populations, even when adjusting for the severity of critical illness.30,31
Several studies of neonates with NEC and AKI have also reported significantly higher mortality rates among those with AKI.18,19,20 In a study of 39 neonates with NEC, Sanchez et al. reported that 38.5% of the neonates experienced AKI, and those with severe AKI experienced significantly higher mortality (46.7 %). Criss et al. found that 54% of neonates with NEC had AKI and there was a statistically significant hazard of death (44% vs. 25.6%, p = 0.008) (hazard ratio (HR) 2.4, 95% CI 1.2–4.8, p = 0.009) among neonates with AKI.18 Our study did not demonstrate a significant difference in mortality among neonates with and without severe AKI. To assess whether those with any AKI differed in mortality rates to those without AKI, we performed a secondary analysis comparing those without AKI to those with any stage AKI. In our cohort, the neonates with any stage AKI had higher mortality, although the difference did not reach a statistically significant difference. Interestingly, the difference in the frequency of mortality in our cohort between those with and without AKI was 10.7%, which is similar to the mortality difference of neonates with and without AKI (10% vs. 1%), reported by AWAKEN investigators.
Our study also demonstrated that neonates had significant hyponatremia within the first 24 h after NEC onset, which may be due to fluid overload as a result of reduced kidney function and oliguria. This theory is supported by the trend of higher SCr and lower UOP during the first 48 h after NEC onset, although the percentage of weight change does not suggest this change in our study population. The serum sodium levels were normalized 48 h after NEC onset due likely to improved UOP and more appropriate tubular handling of fluid and electrolyte balance.
In our cohort, infants with severe AKI were significantly more likely to have had an ostomy (ileostomy, colostomy, or jejunostomy). We suspect that ostomy placement may have been associated with greater severity of inflammation and illness, splanchnic dilatation, and subsequent kidney hypoperfusion (pre-renal).1,32 We also noted a significantly higher number of infants with cholestasis and severe AKI in our study. This may be related to the combination of lower GA and BW, and/or greater frequency of ostomy placement among infants with severe AKI. All of those variables may have contributed to the need for prolonged total parental nutrition, thereby increasing the risk for cholestasis.
Animal studies have demonstrated that NEC is associated with widespread kidney inflammation and interstitial infiltration of glomeruli and tubules. In particular, we have previously published a study that demonstrated an increased expression of proinflammatory markers, such as nuclear factor kappa-light-chain-enhancer of activated B cells, anti-inflammatory markers such as tissue growth factor-beta, and extracellular signal-regulated protein kinase 1/2, along with water- and sodium-regulating proteins, such as claudin-1, -2, -3, -4, -8, and aquaporin-2 in NEC kidneys. This pathophysiologic process known to occur after NEC likely contributes to the greater severity of AKI in our cohort compared to the AWAKEN cohort.11
There was a disproportionately greater number of African-American neonates with NEC and AKI in this cohort compared to the distribution of African-American neonates in the United States. This may be related to the population majority in the Mississippi region, but could also be related to social determinants of health and/or a genetic predisposition [i.e., apolipoprotein L1 (APOL1) gene variants] to developing kidney disease, perhaps making African-American neonates more susceptible to kidney insult.33,34,35 Future studies investigating the role of social determinants of health, race, and APOL1 genetic variants, and the impact on neonatal AKI are needed.
The strengths of this study include that it is the only study evaluating NEC and neonatal AKI using both SCr and UOP. This approach supported greater recognition of AKI that would have been missed if only SCr criteria had been used. We were also able to characterize trends in SCr, UOP, serum sodium, and weight gain up to 2 weeks of NEC, which provides useful clinical information to bedside clinicians and is a platform for further research regarding neonatal AKI clinical trends. Despite these strengths, we acknowledge the following limitations. First, our study is limited by its single-center, retrospective design. The relatively small sample size reduces the generalizability of the study and the statistical power to detect associations between clinical factors, NEC, and AKI. Second, although we suspect neonates in this study experienced AKI due to NEC exposure and the downstream effects of renal hypoperfusion, AKI can often be caused by multiple variables at once. We characterized AKI within 2 weeks after NEC diagnosis, and thus it is plausible that nephrotoxic antibiotics or other unstudied factors could have contributed to AKI diagnoses. Third, we examined entire hospital length of stay, including the time before NEC diagnosis, which may have introduced time bias given that lower GA neonates are more likely to experience longer hospitalizations, although examination of mean index time from birth until NEC diagnosis among neonates with and without severe AKI in our cohort was similar. We also acknowledge the limitations of measuring accurate and comparable weights in the immediate postoperative period given the potential for surgical changes in mass and difficulty with the collection of weight measurements in critically ill neonates. Lastly, there are limitations inherent to using diaper weights to reflect UOP (i.e., inaccurately reflecting UOP if mixed output in the diaper).
In conclusion, our study demonstrates that severe AKI after NEC is common and associated with prolonged hospital length of stay. In our cohort, surgical NEC, outborn status, exposure to antenatal steroids, and positive blood culture sepsis at the time of NEC onset had increased independent odds for severe AKI. In the future, prospective multicenter studies, which allow the inclusion of additional clinical details (e.g., blood pressures) and laboratory predictors such as urinary biomarkers, may support earlier recognition of AKI or identify additional risk factors for AKI after NEC. While some of these exposures are non-modifiable or unavoidable in the setting of an NEC diagnosis, this does highlight the importance of judicious use of nephrotoxic agents in neonates diagnosed with NEC given the higher risk for AKI. Studies that evaluate kidney-protective strategies to prevent AKI and its consequences are greatly needed in the hopes that it will improve the postoperative recovery and clinical outcomes in neonates with NEC.
References
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011).
Sankaran, K. et al. Variations in incidence of necrotizing enterocolitis in Canadian neonatal intensive care units. J. Pediatr. Gastroenterol. Nutr. 39, 366–372 (2004).
Sjoberg Bexelius, T. et al. Intestinal failure after necrotising enterocolitis: incidence and risk factors in a Swedish population-based longitudinal study. BMJ Paediatr. Open 2, e000316 (2018).
Allin, B. S. R. et al. One-year outcomes following surgery for necrotising enterocolitis: a UK-wide cohort study. Arch. Dis. Child Fetal Neonatal Ed. 103, F461–f466 (2018).
Knell, J. et al. Current status of necrotizing enterocolitis. Curr. Probl. Surg. 56, 11–38 (2019).
Stoll, B. J. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 314, 1039–1051 (2015).
Santulli, T. V. et al. Acute necrotizing enterocolitis in infancy: a review of 64 cases. Pediatrics 55, 376–387 (1975).
Mowitz, M. E., Dukhovny, D. & Zupancic, J. A. F. The cost of necrotizing enterocolitis in premature infants. Semin. Fetal Neonatal Med. 23, 416–419 (2018).
Ganapathy, V. et al. Long term healthcare costs of infants who survived neonatal necrotizing enterocolitis: a retrospective longitudinal study among infants enrolled in Texas Medicaid. BMC Pediatr. 13, 127 (2013).
Bao, Y. W. et al. Kidney disease models: tools to identify mechanisms and potential therapeutic targets. Zool. Res. 39, 72–86 (2018).
Garg, P. M. et al. Necrotizing enterocolitis in a mouse model leads to widespread renal inflammation, acute kidney injury, and disruption of renal tight junction proteins. Pediatr. Res. 78, 527–532 (2015).
Jetton, J. G. et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc. Health 1, 184–194 (2017).
Charlton, J. R. et al. Incidence and risk factors of early onset neonatal AKI. Clin. J. Am. Soc. Nephrol. 14, 184–195 (2019).
Wu, Y. et al. Incidence, risk factors, and outcomes of acute kidney injury in neonates after surgical procedures. Pediatr. Nephrol. 35, 1341–1346 (2020).
Mallory, P. P. et al. Acute kidney injury, fluid overload, and outcomes in children supported with extracorporeal membrane oxygenation for a respiratory indication. Asaio J. 66, 319–326 (2020).
Starr, M. C. et al. Acute kidney injury and bronchopulmonary dysplasia in premature neonates born less than 32 weeks’ gestation. Am. J. Perinatol. 37, 341–348 (2020).
Stoops, C. et al. The association of intraventricular hemorrhage and acute kidney injury in premature infants from the Assessment of the Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) Study. Neonatology 116, 321–330 (2019).
Criss, C. N. et al. Acute kidney injury in necrotizing enterocolitis predicts mortality. Pediatr. Nephrol. 33, 503–510 (2018).
Bakhoum, C. Y. et al. Acute kidney injury in preterm infants with necrotizing enterocolitis. J. Matern. Fetal Neonatal Med. 32, 3185–3190 (2019).
Sanchez, C., Garcia, M. A. & Valdes, B. D. Acute kidney injury in newborns with necrotizing enterocolitis: risk factors and mortality. Bol. Med Hosp. Infant Mex. 76, 210–214 (2019).
Bell, M. J. et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 187, 1–7 (1978).
Lambert, D. K. et al. Fulminant necrotizing enterocolitis in a multihospital healthcare system. J. Perinatol. 32, 194–198 (2012).
Selewski, D. T. et al. Neonatal acute kidney injury. Pediatrics 136, e463–e473 (2015).
Jetton, J. G. et al. Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates: design of a Retrospective Cohort Study. Front. Pediatr. 4, 68 (2016).
Jetton, J. G. & Askenazi, D. J. Acute kidney injury in the neonate. Clin. Perinatol. 41, 487–502 (2014).
Zappitelli, M. et al. Developing a neonatal acute kidney injury research definition: a report from the NIDDK neonatal AKI workshop. Pediatr. Res. 82, 569–573 (2017).
Basu, R. K. et al. Assessment of Worldwide Acute Kidney Injury, Renal Angina and Epidemiology in Critically Ill Children (AWARE): a prospective study to improve diagnostic precision. J. Clin. Trials 5, 222 (2015).
Kaddourah, A. et al. Epidemiology of acute kidney injury in critically ill children and young adults. N. Engl. J. Med. 376, 11–20 (2017).
Salerno, S. N. et al. Association between nephrotoxic drug combinations and acute kidney injury in the neonatal intensive care unit. J. Pediatr. S0022-3476(20)31021-0 https://doi.org/10.1016/j.jpeds.2020.08.035 (2020).
Alkandari, O. et al. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit. Care 15, R146 (2011).
Kirkley, M. J. et al. Acute kidney injury in neonatal encephalopathy: an evaluation of the AWAKEN database. Pediatr. Nephrol. 34, 169–176 (2019).
Hashem, R. H. et al. Doppler ultrasound assessment of the splanchnic circulation in preterms with neonatal sepsis at risk for necrotizing enterocolitis. J. Ultrasound 20, 59–67 (2017).
Robertson, C. C. et al. An investigation of APOL1 risk genotypes and preterm birth in African American population cohorts. Nephrol. Dial. Transplant. 32, 2051–2058 (2017).
Preterm Birth. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm (2018).
South, A. M. et al. Renal function and blood pressure are altered in adolescents born preterm. Pediatr. Nephrol. 34, 137–144 (2019).
Acknowledgements
Dr. Keia Sanderson is supported by Grant 2015213 from the Doris Duke Charitable Foundation.
Author information
Authors and Affiliations
Contributions
P.M.G. designed the study; P.M.G., A.B.B., S.S., D.K.B., J.L.P., N.B.O., M.A.Y.A., K.R.S., and D.A. collected and analyzed the data; P.M.G., D.A., M.A.Y.A., and K.R.S. wrote the manuscript. All the authors contributed to and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Garg, P.M., Britt, A.B., Ansari, M.A.Y. et al. Severe acute kidney injury in neonates with necrotizing enterocolitis: risk factors and outcomes. Pediatr Res 90, 642–649 (2021). https://doi.org/10.1038/s41390-020-01320-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01320-6
This article is cited by
-
Risk factor analysis and nomogram prediction model construction for NEC complicated by intestinal perforation
BMC Pediatrics (2024)
-
Outcomes by disease onset, sex, and intervention in neonates with SIP and surgical NEC
Pediatric Research (2024)
-
Perinatal risk factors associated with acute kidney injury severity and duration among infants born extremely preterm
Pediatric Research (2024)
-
Describing patterns in serum creatinine in infants with and without necrotizing enterocolitis
Journal of Perinatology (2023)
-
Gestational age-specific clinical correlates of acute kidney injury in preterm infants with necrotizing enterocolitis
Pediatric Research (2023)